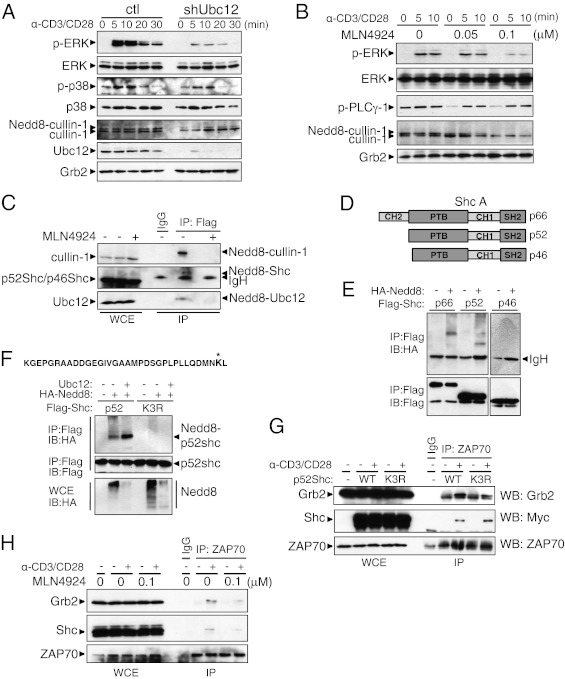
Fig. 3.
The neddylation pathway is involved in Erk activation. (A) Immunoblot analysis of control (Left) and shUbc12 (Right) CD4+ T cells. The CD4+ T cells sorted from the chimeric mice were stimulated with anti-CD3 and anti-CD28 for the indicated time periods. Cell lysates were separated by SDS/PAGE and immunoblotted with the indicated antibodies. (B) CD4+ T cells were preactivated with anti-CD3 in the presence of the indicated concentrations of MLN4924 for 16 h and then restimulated with a combination of anti-CD3 and anti-CD28. (C) Jurkat E6.1 cells stably expressing FLAG-NEDD8 were treated with MLN4924 for 12 h or left untreated before being collected. Cell lysates were boiled in 1% SDS-containing lysis buffer, then diluted to 0.1% SDS and immunoprecipitated with anti-FLAG–conjugated beads, followed by elution with triple FLAG peptide. The eluates were subjected to Western blot analysis using the indicated antibodies. (D) The three isoforms of Shc. All isoforms contain an N-terminal PTB domain, a CH domain, and a C-terminal SH2 domain. (E) Jurkat cells were transfected with FLAG-tagged p66, p52, or p46 with HA-tagged NEDD8. The samples were processed for immunoprecipitation as described in C. (F) The peptide sequence at the top is derived from MS analysis of p66Shc; an asterisk indicates the modified lysine residue. Jurkat cells were transfected with expression vector encoding WT (wt) p52Shc or p52Shc lysine mutant (K3R). Where indicated, combinations of plasmids expressing HA-NEDD8 and/or Ubc12 were cotransfected. (G) CD4+ T cells were stimulated with anti-CD3/CD28 antibodies and retrovirally transduced with Myc-tagged p52Shc WT-IRES-GFP (WT) or Myc-tagged p52Shc K3R-IRES-GFP (K3R). Whole-cell lysates were immunoprecipitated with anti-ZAP70 antibody and blotted with the indicated antibodies. (H) CD4+ T cells were treated with MLN4924 for 16 h or left untreated. After TCR stimulation, the cell lysates were subjected to coimmunoprecipitation with anti-ZAP70 antibody.