Abstract
We recently have introduced the term vasculogenic mimicry to describe the unique ability of aggressive melanoma tumor cells to form tubular structures and patterned networks in three-dimensional culture, which “mimics” embryonic vasculogenic networks formed by differentiating endothelial cells. In the current study, we address the biological significance of several endothelial-associated molecules (revealed by microarray analysis) with respect to expression and function in highly aggressive and poorly aggressive human cutaneous melanoma cell lines (established from the same patient). In a comparative analysis, CD31 was not expressed by any of the melanoma cell lines, whereas TIE-1 (tyrosine kinase with Ig and epidermal growth factor homology domains-1) was strongly expressed in the highly aggressive tumor cells with a low level of expression in one of the poorly aggressive cell lines. Vascular endothelial (VE)-cadherin was exclusively expressed by highly aggressive melanoma cells and was undetectable in the poorly aggressive tumor cells, suggesting the possibility of a vasculogenic switch. Down-regulation of VE-cadherin expression in the aggressive melanoma cells abrogated their ability to form vasculogenic networks and directly tested the hypothesis that VE-cadherin is critical in melanoma vasculogenic mimicry. These results highlight the plasticity of aggressive melanoma cells and call into question their possible genetic reversion to an embryonic phenotype. This finding could pose a significant clinical challenge in targeting tumor cells that may masquerade as circulating endothelial cells or other embryonic-like stem cells.
During embryonic development, the formation of primary vascular networks occurs by vasculogenesis, the in situ differentiation of mesodermal progenitor cells (angioblasts or hemangioblasts) to endothelial cells that organize into a primitive network (for review, see ref. 1). The subsequent growth and remodeling of the vasculogenic primitive network into a more refined complex of functionally efficient vasculature occurs through angiogenesis, the sprouting of new capillaries from a preexisting network (for review, see ref. 2). Similarly, it is widely held that during cancer progression, the blood supply required for tumors to survive, grow, and metastasize occurs via tumor angiogenesis, involving the process of signaling new blood vessel growth into a growing tumor mass (2–5).
Recently, our laboratory and collaborators have introduced the term vasculogenic mimicry to describe the unique ability of aggressive melanoma tumor cells to form tubular structures and patterned networks in three-dimensional culture, which “mimics” the pattern of embryonic vasculogenic networks (6). This initial study used a multidisciplinary approach to investigate the pathological indices in uveal melanoma patient tumors correlated with data generated from tumor-derived cell lines in three-dimensional in vitro cultures, invasion assays, and microarray analysis of differential gene expression. Most noteworthy was the revelation that the presence of matrix-rich networks (surrounding spheroids of tumor cells) in aggressive primary and metastatic uveal melanoma tumors correlated with poor clinical outcome in patients (6, 7). Furthermore, the cell lines derived from the highly aggressive, but not poorly aggressive, tumors, were invasive in vitro and capable of forming embryonic-like vasculogenic networks in three-dimensional culture. Microarray analysis of differential gene expression of highly aggressive vs. poorly aggressive human uveal (6) and cutaneous (8) melanoma cell lines revealed the coexpression of multiple phenotypic markers by the aggressive tumor cells that are characteristic of endothelial, epithelial, and hematopoietic phenotypes, collectively suggesting a genetic reversion to a pluripotent, embryonic-like phenotype. However, the biological significance of these aberrantly expressed genes and the molecular mechanisms involved in vasculogenic mimicry remain enigmatic.
In the current study, we addressed the biological significance of several endothelial-associated molecules with respect to expression and function in highly aggressive and poorly aggressive human cutaneous melanoma cell lines established from the same patient. One of the endothelial-specific genes that was expressed by aggressive melanoma cells with highest fidelity is vascular endothelial (VE)-cadherin (CD144 or cadherin 5). VE-cadherin is an adhesive protein, known to be expressed exclusively by endothelial cells, and belongs to the cadherin family of transmembrane proteins promoting homotypic cell-to-cell interaction (9–12). The significance of VE-cadherin is exemplified by reports showing that mice deficient in VE-cadherin or expressing truncated VE-cadherin die in midgestation from severe vascular defects, involving endothelial apoptosis and disrupted survival signaling pathways (13). In the present study, VE-cadherin is shown to be exclusively expressed by aggressive human cutaneous and uveal melanoma tumor cells and not expressed by the poorly aggressive tumor cells. Furthermore, the biological significance of this finding is demonstrated through knockout experiments of VE-cadherin, which resulted in the inability of the aggressive melanoma cells to form embryonic-like vasculogenic networks in three-dimensional culture. An additional cell adhesion protein, CD31, a platelet endothelial cell adhesion molecule, was not expressed by any of the melanoma tumor cells. TIE-1 (tyrosine kinase with Ig and epidermal growth factor homology domains-1), a receptor tyrosine kinase, was strongly expressed in the highly aggressive tumor cells with a very low level of expression in a poorly aggressive uveal melanoma cell line. These studies highlight the plasticity of aggressive human melanoma tumor cells and call into question the underlying significance of their ability to express an endothelial-specific molecule critical in the formation of vasculogenic networks and vasculogenic mimicry.
Materials and Methods
Cell Culture.
The human cutaneous (C81–61 and C8161) and uveal melanoma (OCM-1A, C918, MUM-2B, and MUM-2C) cell lines have been characterized previously according to their phenotype, invasive and metastatic potential, and clinical significance. C8161, C918, and MUM-2B cells are highly aggressive, whereas C81–61, OCM-1A, and MUM-2C are poorly aggressive melanoma cells (6, 14, 15). Human umbilical vein endothelial cells (HUVECs) were maintained in DMEM, 10% FBS, 1× Mito+ (Collaborative Biomedical, Bedford, MA), and 0.1% gentamicin sulfate. Angioblasts containing CD34− leukocytes were isolated and cultured as described for CD34+ enriched angioblasts, then fixed and photographed after 24 days in culture (16).
Three-dimensional type I collagen gels were produced with 15 μl of type I collagen (average 3 mg/ml; Collaborative Biomedical) on 18-mm glass coverslips and analyzed by phase and transmission electron microscopy, as described (6).
Microarray Analysis.
cDNA microarrays detected altered gene expression in highly invasive human cutaneous melanoma cells by the method previously described (8), and the relative expression of selected genes critical for vascular formation and maintenance is reported as highly invasive and metastatic compared with poorly invasive melanoma cells (Table 1). Hybridization to cDNA microarrays and data analysis followed the previously described procedures (ref. 8; also see http://www.nhgri.nih.gov/DIR/LCG/15K/HTML/).
Table 1.
Microarray analysis of endothelial-associated genes expressed by cutaneous melanoma
| Protein | Symbol/unigene | Function | Ratio* |
|---|---|---|---|
| CD31 | PECAM/Hs.78146 | Adhesion molecule | 0.2 |
| TIE-1 | TIE/Hs.78824 | Receptor tyrosine kinase | 25.0 |
| VE-cadherin | CDH5/Hs.76206 | Cell–cell adhesion | 10.7 |
cDNA microarray analysis was performed as described (8), comparing highly aggressive (C8161) vs. poorly aggressive (C81-61) human cutaneous melanoma cells from the same patient. Approximately 1,600 expressed sequence tags and known genes were differentially expressed from a total of 6,000.
Western Blot Analysis.
The protein concentration in whole-cell lysates from the different cultures were determined by using a microbicinchoninic acid protein assay (BCA, Pierce) and equal amounts of protein for each cell line loaded onto 7.5% reducing sodium-dodecylsulfate polyacrylamide gels for electrophoresis. The separated proteins were transblotted onto Immobilon-P membranes (Millipore), which subsequently were probed with antibodies to human VE-cadherin (Transduction Laboratories, Lexington, KY), CD-31, or TIE-1 (R&D Systems), and detected as described (14).
Semiquantitative Reverse Transcription (RT)-PCR Analysis.
RT of total RNA from HUVECs and melanoma cell lines was performed by using the Advantage PCR kit as per the manufacturer's instructions (CLONTECH). PCR amplification reactions using VE-cadherin-specific primers (forward: 5′-CCGGCGCCAAAAGAGAGA-3′; reverse: 5′-CTGGTTTTCCTTCAGCTGGAAGTGGT-3′) were performed in a Robocycler Gradient 96 Thermocycler (Stratagene): 1 cycle: 94°C, 1 min; 30 cycles: 94°C, 1 min; 68°C, 2.5 min, 72°C, 1 min; and 1 cycle: 72°C, 5 min. Glyceraldehyde-3-phosphate dehydrogenase primers (CLONTECH) were used as controls for PCR amplification. PCR fragments were verified by DNA sequencing analysis and showed 100% identity to VE-cadherin cDNA sequence.
Immunohistochemistry.
The identification and localization of VE-cadherin and CD-31 in three-dimensional cell cultures was performed by using antibodies to VE-cadherin (Transduction Laboratories) and CD-31 (R&D Systems) and the Vectastain ABC and AEC kits (Vector Laboratories; 40×). Briefly, cultures were fixed in 3.7% formaldehyde followed by a wash with 0.1% Triton X-100 in PBS for 5 min. The cultures then were incubated with the primary antibody for 2 h followed by processing using the Vectastain ABC and AEC kits according to the manufacturer's protocol, or for immunofluorescence microscopy, as described (15). Images were obtained by using a Zeiss Axioskop 2, equipped with a Spot 2 camera system (Diagnostic Instruments, Sterling Heights, MI), and images were captured by using the Zeiss AXIOVISION 2.0.5 software package.
Microinjection of Fluoresceinated Dyes.
Patterned tubular networks observed in 3-week-old cultures of C8161 human cutaneous melanoma cells grown on collagen I gels were injected with 7% lucifer yellow dye in water (wt/vol; lithium salt, Molecular Probes) by using a Zeiss 135 Axiovert microscope and Eppendorf 5170 micromanipulator and 5242 microinjector (Brinkmann). A video recording was made by using an Optronics camera (Goleta, CA) and videocassette recorder, and images subsequently were captured from the tape by using a Matrox Meteor frame grabber (Dorval, Canada) and axiovision software.
Knockout of VE-Cadherin.
The surface availability and/or expression of VE-cadherin on highly aggressive C8161 human melanoma cells was transiently suppressed by treating the cells with a blocking mAb to VE-cadherin (cadherin-5; Transduction Laboratories; 2.5 μg/ml final concentration) alone, or by phosphorothioate-modified oligonucleotides (commercially prepared by Integrated DNA Technologies; Coralville, IA) as scrambled (5′-3′; GACTGGAATGCAGATCGA) or in the sense (5′-3′; AAGATGCAGAGGCTCATG; 18-mer) or antisense (5′-3′; CATGAGCCTCTGCATCTT; 18-mer) orientations (or a combination of both oligonucleotides and antibody application). Treated cells were seeded onto 18-mm coverslips coated with a three-dimensional collagen I matrix in a 12-well dish (5 × 105 cells/well in 1.0 ml complete medium). The cells were allowed to attach for 3 h, washed once with serum-free Opti-MEM medium (Life Technologies, Gaithersburg, MD) and incubated for 5 h with 1 μM scrambled, sense, or antisense oligonucleotides by using the Lipofectamine delivery system (Life Technologies). Serum-containing medium was added to the cells, and fresh oligonucleotides were added every other day (or fresh antibody daily). At 1 week postseeding, the medium was removed and the cells were washed with 1× PBS. For confirmation of VE-cadherin knockout, the cells were subjected to Western blot analysis. Confirmation that the treatment inhibited the formation of the matrix patterns was performed by fixing the cultures with 3.7% formaldehyde, then staining the cultures with a periodic acid-Schiff without the hemotoxylin counterstain.
Results
VE-Cadherin Is Exclusively Expressed by Aggressive Melanoma Cells.
Microarray analysis of poorly aggressive vs. highly aggressive human cutaneous melanoma cells (derived from the same patient) by using hybridization to cDNA microarrays allowed for the direct comparison of the differential expression of 6,000 genes simultaneously. From this analysis, as illustrated in Table 1, two endothelial-specific genes are shown to be overexpressed in the highly aggressive cutaneous melanoma cells compared with the poorly aggressive cells derived from the same patient. Specifically, TIE-1 (a receptor tyrosine kinase) and VE-cadherin (CD144 or cadherin 5) were significantly overexpressed by the highly aggressive melanoma cells. In contrast, CD31 (a platelet endothelial cell adhesion molecule; PECAM-1) was not differentially expressed.
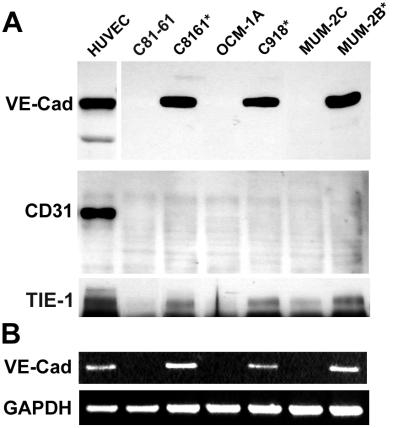
Further investigation of the differential expression of select endothelial-specific genes was achieved by Western blot analysis, as shown in Fig. 1. Using an expanded panel of highly and poorly aggressive human cutaneous and uveal melanoma cell lines, the expression of VE-cadherin, TIE-1, and CD31 was probed with commercially available mAbs to each respective endothelial-specific protein. The Western data (Fig. 1A) confirmed the microarray expression patterns of these endothelial-specific genes at the protein level for VE-cadherin and TIE-1, as well as the absence of expression of CD31 in all melanoma cell lines tested. The HUVECs served as a positive control for VE-cadherin and CD31 expression. RT-PCR analysis further validated the VE-cadherin expression profile in melanoma cell lines (Fig. 1B). Most noteworthy was the strong expression of VE-cadherin by the aggressive melanoma cells and the complete absence of expression by the poorly aggressive cells.
Figure 1.
Western blot (A) and semiquantitative RT-PCR (B) analyses of endothelial-associated markers by human melanoma tumor cells. Western blot analysis of VE-cadherin (VE-cad), CD31, and TIE-1 in human cutaneous and uveal melanoma highly aggressive (*) and poorly aggressive cell lines (A). RT-PCR analysis of VE-cadherin (VE-cad) expression in similar melanoma cell samples as shown in A. Equal loading is demonstrated by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression (B). The aggressive melanoma cells form primitive vasculogenic networks in three-dimensional collagen gels in vitro. (HUVECs were used as positive controls for VE-cadherin expression.)
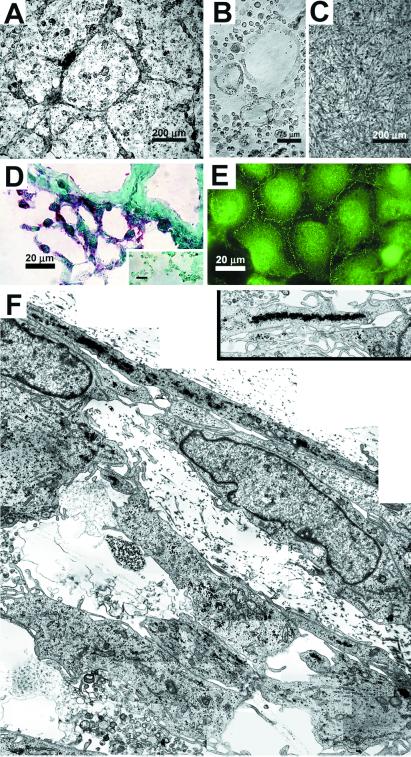
Functional studies were performed to test the hypothesis that VE-cadherin expression by aggressive melanoma tumor cells resulted in their ability to mimic endothelial cells by forming patterned vasculogenic networks. Morphological analyses of human cutaneous melanoma cells in three-dimensional culture after 3 days revealed that highly aggressive C8161 cells formed a vasculogenic pattern of cord-like networks (Fig. 2A), similar to those formed de novo by angioblasts (Fig. 2B). Poorly aggressive C81–61 cells were unable to form networks under similar culture conditions (Fig. 2C). Immunohistochemical analysis of VE-cadherin distribution in cryostat sections of the C8161-formed networks (similar to those shown in Fig. 2A) demonstrated a red chromogenic stain outlining the network structures throughout the culture (Fig. 2D). The negative control receiving no primary antibody showed no nonspecific staining by the secondary antibody (Fig. 2D Inset). Localization of the VE-cadherin in monolayer culture of C8161 cells highlighted the specific labeling of cell adhesion junctions (Fig. 2E). Transmission electron microscopic analysis (Fig. 2F) of developing melanoma cell networks (in three-dimensional culture), formed by aggressive cutaneous C8161 melanoma cells, illustrates the flattened, endothelial-like nature of the outermost perimeter cells, and some evidence of cellular debris (possibly due to apoptosis), during the establishment and remodeling of what will become larger tubular networks (demonstrated in Fig. 3A). Endothelial-like fenestrae are also evident. The higher-resolution transmission electron micrograph inset in Fig. 2F illustrates the alignment of melanosomes within the melanoma cells forming the perimeter of a network.
Figure 2.
Morphological and immunohistochemical analyses of highly aggressive and poorly aggressive cutaneous melanoma cells in three-dimensional collagen gels (A–D and F) and in monolayer culture (E). Phase-contrast microscopy (A–C) demonstrates the ability of highly aggressive cutaneous C8161 melanoma cells to form a vasculogenic pattern of cordlike networks (A), similar to those formed de novo by human angioblasts (B). Poorly aggressive cutaneous C81–61 melanoma cells were unable to form networks under similar culture conditions (C). Immunohistochemical staining for VE-cadherin (shown as red chromogenic stain) in a cryostat section of C8161 cells highlights developing vasculogenic networks; the negative control (Inset) receiving no primary antibody showed no nonspecific staining by the secondary antibody (D). Immunostaining for VE-cadherin in C8161 monolayer culture demonstrates the intercellular junctions (E). Transmission electron microscopy of developing vasculogenic networks by C8161 cells reveals the endothelial-like nature of the outermost perimeter cells (F). Alignment of melanosomes within the perimeter network cells is shown in the Inset.
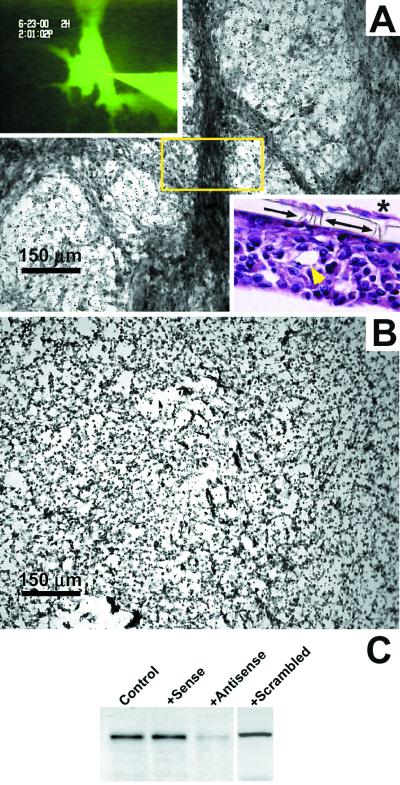
Figure 3.
Morphological and biochemical analyses of VE-cadherin knockout in highly aggressive human cutaneous C8161 melanoma cells. Cells were treated with sense (A) or antisense (B) oligonucleotides to VE-cadherin over 1 week in three-dimensional collagen gels, followed by periodic acid-Schiff staining to highlight extracellular matrix-rich vasculogenic networks. (This method renders tumor cells not easily detectable.) The tumor cell cultures receiving the control sense (A) or scrambled oligonucleotides (not shown) formed tubular networks that were perfusable with microinjected fluorescent dye (Upper Left Inset; from the tubular region in the box) and appeared as hollow tubes with lumens in longitudinal section (yellow arrowhead; Lower Right Inset). The Lower Right Inset also demonstrates the invasive ability of the C8161 cells (*) to migrate through the collagen gel and the 10-μm size pores (arrows) in the polycarbonate filter to the undersurface of the filter where they form a monolayer in the absence of a matrix. The cultures receiving the antisense oligonucleotide treatment did not form networks (B). Western blot analysis (C) of VE-cadherin expression shows the diminution of VE-cadherin in the antisense-treated cells, but not in the controls (no treatment), sense-treated, or scrambled oligonucleotide-treated cells.
Down-Regulation of VE-Cadherin Abrogates Vasculogenic Mimicry.
Further experiments were designed to directly test the hypothesis that VE-cadherin was critical to the formation of melanoma-generated networks, using antisense oligonucleotides to down-regulate the expression of VE-cadherin. Microinjection of three-dimensional cultures of aggressive melanoma cell-formed networks revealed their ability to conduct a fluorescent dye (Fig. 3A Upper Left Inset), thus indicating the hollow nature of the mature tubular networks. A longitudinal section through the melanoma networks in the three-dimensional collagen gel illustrates several hollow tubes, in addition to the invasive ability of C8161 cells to migrate through the 10-μm size pores in the polycarbonate filter to the undersurface of the filter where they form a monolayer in the absence of a gel matrix (Fig. 3A Lower Right Inset). Aggressive melanoma cells treated with sense (Fig. 3A), scrambled sequence (not shown), or antisense (Fig. 3B) oligonucleotides to VE-cadherin demonstrated that no networks could form when VE-cadherin expression was down-regulated (Fig. 3B). Periodic acid-Schiff stain was applied to these cultures to highlight the extracellular matrix composition of the networks, as described (6), which does not render cells easily detectable. VE-cadherin expression after no treatment or sense, antisense, or scrambled oligonucleotide treatment, was verified by Western blot analysis (Fig. 3C).
Discussion
Attempts to elucidate the mechanisms of blood supply to growing tumors have presented a significant challenge using detection methods based on traditional markers of endothelial-specific vs. tumor-specific antigens. The introduction of the concept of angiogenesis-driven tumor growth has ignited a cottage industry of research, which forms the basis for new and hopeful therapies targeted at endothelial-lined tumor vasculature (3). Equally important has been the application of microarray analyses, hereby providing new molecular classifications of tumors based on gene expression profiles (8). However, from a clinical perspective, it remains enigmatic why some tumors are able to respond to certain cancer therapies, whereas others do not. Possibly, the answer to this question could be better addressed by first considering the “embryonic footprint” of the cancer cells comprising specific tumors.
As we have learned from the study of embryogenesis, the process responsible for the establishment of primary vascular networks is vasculogenesis. This initial process involves the in situ differentiation of undifferentiated mesenchymal precursor cells to endothelial cells that are programmed to organize a primitive network (1, 2). In fact, new evidence suggests that these endothelial cells can be derived from angioblasts or hemangioblasts, and that a newly discovered vascular progenitor cell can produce both smooth muscle cells and endothelial cells (17). The remodeling of the primitive vasculogenic network occurs via angiogenesis, and although it is assumed that the development of new vessels in the adult arises primarily from the angiogenic process, vasculogenesis has been reported in certain adult pathological conditions as well (1).
Our study provides strong evidence in support of the hypothesis that expression of VE-cadherin by aggressive melanoma tumor cells results in their ability to mimic endothelial cells and form embryonic-like, patterned, vasculogenic networks in three-dimensional culture, hence the term vasculogenic mimicry. The microarray data, comparing the gene expression of highly aggressive (C8161) vs. poorly aggressive (C81–61) human cutaneous melanoma cells (derived from the same patient), revealed the differential expression of ≈1,600 expressed sequence tags and known genes from a total of 6,000 examined and suggested the genetic reversion of the aggressive melanoma cells to an embryonic-like phenotype (8). This assumption is based on the aberrant expression by aggressive melanoma cells of genes associated with multiple phenotypes including endothelial cells, as well as hematopoietic stem cells. We focused our study on three of the endothelial-specific genes (TIE-1, CD31, and VE-cadherin) to elucidate their potential involvement in tumor cell vasculogenic mimicry. All three of these genes have been shown to play a key role in endothelial blood vessel formation and function (1); however, their expression patterns at the protein level were quite disparate. The platelet endothelial cell adhesion molecule CD31 was not expressed by any of the melanoma tumor cells tested. The receptor tyrosine kinase TIE-1 was strongly expressed in the highly aggressive cutaneous and uveal melanoma cells and was not detected in the poorly aggressive cutaneous melanoma cells. However, the poorly aggressive uveal melanoma cells expressed a low level of TIE-1. The expression of VE-cadherin was strong in the highly aggressive melanoma cells and undetectable in the poorly aggressive melanoma cells. The VE-cadherin expression pattern demonstrated by the matched pairs of human melanoma cells tested in this study may represent a “vasculogenic switch” in the aggressive tumor cells.
The functional analysis of VE-cadherin with respect to tumor cell vasculogenic mimicry revealed a typical cell-to-cell junction localization pattern in the highly aggressive melanoma cells. In three-dimensional culture, the aggressive tumor cells formed a vasculogenic patterned network of cord-like structures, similar to the differentiating angioblasts, their putative precursors. The tumor cell vasculogenic networks stained positively for VE-cadherin; whereas the poorly aggressive tumor cells were unable to form network structures under similar culture conditions. Ultrastructural assessment of the developing vasculogenic networks by aggressive tumor cells demonstrated the presence of flattened and elongated tumor cells at the perimeter of the networks, containing the alignment of melanosomes throughout the cytoplasm. Endothelial-like fenestrae also were detected, and there was evidence of apoptotic debris in the developing lumen. Older melanoma cultures contained well developed tubular structures, which were perfusable with a microinjected fluoresceinated dye. The importance of VE-cadherin in the formation of the vasculogenic networks and tubular structures was directly tested through the use of antisense oligonucleotides to down-regulate VE-cadherin expression.
There is no question that VE-cadherin expression is critical in vasculogenic events. Before this study, VE-cadherin was considered an adhesive transmembrane protein exclusively expressed at interendothelial junctions in differentiated cells (9–13). In fact, deficiency of VE-cadherin or truncation of the gene results in embryonic lethality in transgenic mouse models, induction of endothelial apoptosis, and abolishment of vascular signaling cascades (13, 18). Further evidence highlighting the role of VE-cadherin in vascular structure assembly was reported with the use of targeted null mutation of VE-cadherin in mouse embryonic stem cells, which resulted in the failed organization of “vascular-like structures” in embryoid bodies (19).
Previous studies also have examined the comparative role of VE-cadherin and CD31 with respect to endothelial tubulogenesis in cell culture models. These reports have shown that VE-cadherin-expressing cells are capable of forming tubular networks in three-dimensional gels, but CD31 transfectants could not (20). Further dissection of the functional roles for CD31 and VE-cadherin in endothelial tube assembly and lumen formation in three-dimensional culture revealed that both proteins appeared to be important in cell-to-cell association, but CD31 may be responsible for cell migration or invasion of collagen gels, whereas VE-cadherin may be involved in intercellular lumen-formation events (21). In our study, the lack of CD31 expression in the aggressive melanoma cells apparently did not affect the migratory/invasive ability of these tumor cells, because they had already acquired an invasive phenotye (6).
By microarray analysis, the 25-fold increase in TIE-1 expression by the highly aggressive melanoma cells compared with the poorly aggressive cells suggested that this vascular endothelial receptor tyrosine kinase might play a key role in tumor cell vasculogenic mimicry. However, Western blot analysis revealed a low level of expression of TIE-1 in one of the poorly aggressive uveal melanoma cell lines. Additional experimental data (not presented) demonstrated that treatment of highly aggressive melanoma cells with inhibitory antibodies or antisense oligonucleotides to TIE-1 did not affect the formation of vasculogenic networks, which may suggest its nonessential role in the structural development of cords and tubes. Other studies focused on the distinctive roles of TIE-1 and TIE-2 in blood vessel formation have shown that TIE-1 is essential for vascular endothelial cell integrity, but not critical for the differentiation of hematopoietic cells (22, 23). However, TIE-2 studies have indicated its importance in endothelial cell vascular network formation (22). It is important to consider that during the dynamic process of vascular development, many growth factors, protein tyrosine kinases, and cell adhesion molecules are integrally involved in cooperative interactions (1, 24). Although our current study did not focus on the role of growth factors, it is apparent from the microarray analysis data that many endothelial-associated growth factors and their respective receptors are expressed by the melanoma cells capable of engaging in vasculogenic mimicry. However, in a previous investigation, we determined that the addition of basic fibroblast growth factor, transforming growth factor β, vascular endothelial growth factor, or platelet-derived growth factor to poorly aggressive uveal melanoma cells was not sufficient to induce them to form vasculogenic networks, further supporting the hypothesis that the aggressive melanoma cells express a different genetic program than the poorly aggressive cells (6).
The findings presented in our study demonstrate that aggressive melanoma tumor cells express VE-cadherin, heretofore exclusively associated with endothelial cells. The functional significance of this remarkable finding is reflected by the role VE-cadherin plays in tumor cell formation of embryonic-like, vasculogenic networks de novo. Another intriguing example of a cell type (other than an endothelial cell) expressing VE-cadherin has been reported for cytotrophoblasts (25); they have been shown to adopt a vascular phenotype (called pseudovasculogenesis) as they differentiate in human placental tissue. In the developing murine allantois, vasculogenesis was shown to be independent of erythropoiesis, indicating distinct biological events (26). Based on these collective findings, together with the aberrant expression by aggressive melanoma cells of endothelial-associated genes (8), we have suggested that these melanoma cells are able to engage in vasculogenic mimicry due to their reversion to an embryonic-like phenotype. The two most pressing questions regarding tumor cell vasculogenic mimicry are: Does this phenomenon occur in vivo, and if so, what is its biological significance?
In response to the first question, there is a growing body of in vivo evidence supporting the concept that melanoma tumor cells can line vascular channels, tumor sinuses, and vessel-like spaces (6, 7, 27–29). In addition, mosaic blood vessels (consisting of both tumor cells and endothelial cells) recently have been reported in colon carcinoma; however, of the tumors examined, there was no correlation between the frequency of these vessels and tumor grade/stage (30). Studies of aggressive breast carcinoma have documented the absence of endothelial cells and the lack of central necrosis and have suggested nonangiogenic, as well as angiogenic, pathways of dissemination (31, 32). Unfortunately, one of the major challenges in adequately determining the presence of tumor cells within the tumor vasculature is the selection of detection methods that unequivocally distinguish tumor cells from endothelial cells, smooth muscle cells, or pericytes. Many studies rely on the presence of VE-cadherin to identify endothelial cells. However, the findings presented in this study would refute that approach. The use of CD31 is also popular in the identification of endothelial cells. Again, recent observations in breast and ovarian carcinoma indicate that CD31 is expressed by the aggressive tumor cells (33, 34), thereby questioning its utility as exclusively endothelial cell-specific. Obviously, better detection methods using double-labeling techniques and more specific molecular probes are warranted to address the question of vasculogenic mimicry and mosaic vessels.
The question of biological relevance with respect to vasculogenic mimicry is the most perplexing. The suggestion has been made that tumor plasticity allows vasculogenic mimicry to occur (35). Is this a novel form of an angiogenic or vasculogenic switch? In embryonic development, vasculogenesis and angiogenesis occur concomitantly. Based on the molecular profile of the aggressive melanoma cells, it is tempting to speculate that they have reverted to an embryonic-like phenotype recapitulating the formation of primitive vasculogenic networks. Histological evidence showing a convergence of melanoma tumor-lined and endothelial-lined vasculature suggests a potential morphological pathway for perfusion and dissemination associated with aggressive, but not nonaggressive, tumors (7). However, this supposition requires rigorous follow-up. The most profound implication of the current study rests in the translational impact of targeting aggressive tumor cells that may masquerade as circulating endothelial cells. Although the application of mAb to VE-cadherin inhibits endothelial cell-driven angiogenesis in Lewis lung and epidermoid tumors (36), it remains to be seen whether this therapeutic approach will have widespread utility in other tumors. Clearly, there is a great deal more to understand about the molecular mechanisms underlying VE-cadherin expression in aggressive melanoma cells, and possibly other tumor types, and its role in vasculogenic mimicry.
Acknowledgments
We gratefully acknowledge the participation of Drs. Karla J. Daniels, Robert Folberg, H. Culver Boldt, and Jacob Pe'er in the generation of some of the uveal melanoma cell lines; June Kan Mitchell for the gift of the OCM-1 cells; Julie Buckmeyer and Dr. Frank Meyskens, Jr. for the human cutaneous melanoma cell lines; Kathy Walters and the Iowa Central Microscopy Facility for transmission electron microscopy; and Maged Harraz for preparing the angioblasts. Research was supported by National Institutes of Health Grants CA59702 and CA80318 (to M.J.C.H.), DK55965 (to G.C.S.), and CA83137 (to R.E.B.S.).
Abbreviations
- VE
vascular endothelial
- TIE-1
tyrosine kinase with Ig and epidermal growth factor homology domains-1
- HUVEC
human umbilical vein endothelial cell
- RT
reverse transcription
References
- 1.Carmeliet P. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 2.Risau W. Nature (London) 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. N Eng J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 4.Rak J, Kerbel R S. Cancer Metastasis Rev. 1996;15:231–236. doi: 10.1007/BF00437476. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Fidler I J. In Vivo. 1998;12:27–34. [PubMed] [Google Scholar]
- 6.Maniotis A J, Folberg R, Hess A, Seftor E A, Gardner L M, Pe'er J, Trent J M, Meltzer P S, Hendrix M J C. Am J Pathol. 1999;155:739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folberg R, Hendrix M J C, Maniotis A J. Am J Pathol. 2000;156:361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, et al. Nature (London) 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 9.Hynes R O. Curr Opin Genet Dev. 1992;2:621–624. doi: 10.1016/s0959-437x(05)80182-0. [DOI] [PubMed] [Google Scholar]
- 10.Kemler R. Semin Cell Biol. 1992;3:149–155. doi: 10.1016/s1043-4682(10)80011-x. [DOI] [PubMed] [Google Scholar]
- 11.Lampugnani M G, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco L P, Dejana E. J Cell Biol. 1992;118:1511–1522. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gumbiner B M. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Lampugnani M G, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, et al. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 14.Hendrix M J C, Seftor E A, Chu Y-W, Seftor R E B, Nagle R B, McDaniel K M, Leong S P L, Yohem K H, Leibovitz A M, Meyskens F L, Jr, et al. J Natl Cancer Inst. 1992;84:165–174. doi: 10.1093/jnci/84.3.165. [DOI] [PubMed] [Google Scholar]
- 15.Hendrix M J C, Seftor E A, Seftor R E B, Kirschmann D A, Gardner L M, Boldt H C, Meyer M, Pe'er J, Folberg R. Am J Pathol. 1998;152:855–863. [PMC free article] [PubMed] [Google Scholar]
- 16.Schatteman G C, Hanlon H D, Jiao C, Dodds S G, Christy B A. J Clin Invest. 2000;106:571–578. doi: 10.1172/JCI9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carmeliet P. Nature (London) 2000;408:43–45. doi: 10.1038/35040684. [DOI] [PubMed] [Google Scholar]
- 18.Gory-Faure S, Prandini M H, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P. Development (Cambridge, UK) 1999;126:2093–2102. doi: 10.1242/dev.126.10.2093. [DOI] [PubMed] [Google Scholar]
- 19.Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Proc Natl Acad Sci USA. 1997;94:6273–6278. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halama T, Staffler G, Hoch S, Stockinger H, Wolff K, Petzelbauer P. Int Arch Allergy Immunol. 1999;120:237–244. doi: 10.1159/000024273. [DOI] [PubMed] [Google Scholar]
- 21.Yang S, Graham J, Kahn J W, Schwartz E A, Gerritsen M E. Am J Pathol. 1999;155:887–895. doi: 10.1016/S0002-9440(10)65188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodewald H R, Sato T N. Oncogene. 1996;12:397–404. [PubMed] [Google Scholar]
- 23.Sato T N, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolberg H, Risau W, Qin Y. Nature (London) 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 24.Yancopoulos G D, Davis S, Gale N W, Rudge J S, Wiegand S J, Holash J. Nature (London) 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Y, Fisher S J, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky C H. J Clin Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downs K M, Gifford S, Blahnik M, Gardner R L. Development (Cambridge, UK) 1998;125:4507–4520. doi: 10.1242/dev.125.22.4507. [DOI] [PubMed] [Google Scholar]
- 27.Baron J A, Monzon F, Galaria N, Murphy G F. Hum Pathol. 2000;31:1520–1522. doi: 10.1053/hupa.2000.21063. [DOI] [PubMed] [Google Scholar]
- 28.Tímár J, Tóth J. Pathol Oncol Res. 2000;6:83–86. doi: 10.1007/BF03032354. [DOI] [PubMed] [Google Scholar]
- 29.Warren B A, Shubik P. Lab Invest. 1966;15:464–478. [PubMed] [Google Scholar]
- 30.Chang Y S, di Tomaso E, McDonald D M, Jones R, Jain R K, Munn L L. Proc Natl Acad Sci USA. 2000;97:14608–14613. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirakawa K, Tsuda H, Heike Y, Kato K, Asada R, Inomata M, Sasaki H, Kasumi F, Yoshimoto M, Iwanaga T, et al. Cancer Res. 2001;61:445–451. [PubMed] [Google Scholar]
- 32.Pezzella F, Manzotti M, De Bacco A, Viale G, Nicholson A G, Price R, Ratciffe C, Pastorino U, Gatter K C, Harris A L, et al. Lancet. 2000;355:1787–1788. [Google Scholar]
- 33.Hendrix M J C, Seftor E A, Kirschmann D A, Seftor R E B. Breast Cancer Res. 2000;2:417–422. doi: 10.1186/bcr88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sood A K, Seftor E A, Fletcher M S, Gardner L M G, Heidger P M, Buller R E, Seftor R E B, Hendrix M J C. Am J Pathol. 2001;158:1279–1288. doi: 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bissel M J. Am J Pathol. 1999;155:675–679. doi: 10.1016/S0002-9440(10)65164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao F, Li Y, O'Connor W, Zanetta L, Bassi R, Santiago A, Overholser J, Hooper A, Mignatti P, Dejana E, et al. Cancer Res. 2000;60:6805–6810. [PubMed] [Google Scholar]