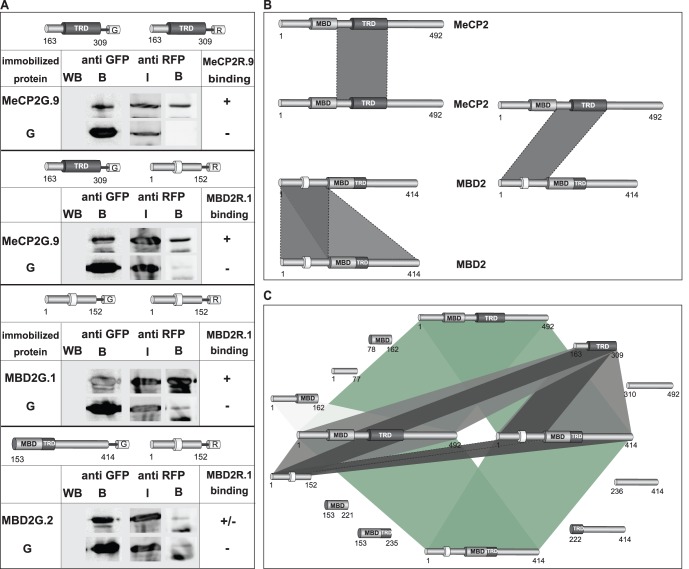
Figure 4. In vivo homo- and hetero-interactions between domains of MeCP2 and MBD2.
(A) GFP- and RFP-tagged domains of MeCP2 and MBD2 as well as GFP control were co-expressed in HEK293-EBNA cells as indicated. After cell lysis using 200 mM NaCl buffer conditions, the extract was incubated with GBP-bound beads for co-immunoprecipitation analysis under the same buffer conditions. The immobilized protein complexes were washed afterwards with the same buffer as used for lysis and co-immunoprecipitation. The immobilized GFP-labeled proteins (B) used for the interaction assay were visualized by western blot using anti GFP antibody. The input (I) and the co-immunoprecipitated fraction (B) of the RFP-labeled proteins were visualized through western blot using anti RFP antibody. The input (I) represents 7% of the total reaction volume. (B and C) Schematic representation of the domains responsible for the homo-and hetero-interactions of MeCP2 and MBD2 (dark grey) illustrating the outcome of the in vivo and in vitro interaction analyses. Numbers stand for amino acid (aa) coordinates. (C) Full-length (fl) MeCP2 and MBD2 directly bind to themselves and each other (green). In case of the MeCP2 homo-interaction, the ID-TRD (aa 163–309) is the domain of MeCP2 that mediates strong direct binding to fl MeCP2 (light grey) and further recognizes the ID-TRD domain independently (dark grey). Regarding MeCP2 and MBD2 hetero-interaction, MeCP2 ID-TRD domain exhibits strong association to fl MBD2 in comparison to other MeCP2 domains (light grey) and further directly and independently interacts to the NH2-terminal domain (NTD, aa 1–152) of MBD2 (dark grey). The NTD is also the only domain of MBD2 that shows strong binding to fl MeCP2 (light grey) and strongly binds to MeCP2 ID-TRD independently (dark grey). In the case of the MBD2 homo-interaction, the NTD is again the region of MBD2 exerting the strongest binding to fl MBD2 (light grey) and further recognizes MBD2 NTD and COOH-terminal domain (dark grey).