Abstract
Although several urinary biomarkers have been validated as early diagnostic markers of acute kidney injury (AKI), their usefulness as outcome predictors is not well established. This study aimed to determine the diagnostic and prognostic abilities of urinary liver-type fatty acid-binding protein (L-FABP) in heterogeneous critically ill patients. We prospectively collected data on patients admitted to medical and surgical intensive care units (ICUs) from July 2010 to June 2011. Urine neutrophil gelatinase-associated lipocalin (NGAL) and L-FABP at the time of ICU admission were quantitated. Of the 145 patients, 54 (37.2%) had AKI defined by the Acute Kidney Injury Network (AKIN) criteria. AKI patients showed significantly higher level of urinary NGAL and L-FABP and also higher mortality than non-AKI patients. The diagnostic performances, assessed by the area under the ROC curve, were 0.773 for NGAL and 0.780 for L-FABP, demonstrating their usefulness in diagnosing AKI. In multivariate Cox analysis, urinary L-FABP was an independent predictor for 90-day mortality. Urinary L-FABP seems to be promising both for the diagnosis of AKI and for the prediction of prognosis in heterogeneous ICU patients. It needs to be further validated for clinical utility.
Keywords: Acute Kidney Injury, Liver-Type Fatty Acid-Binding Protein, Neutrophil Gelatinase-Associated Lipocalin, Prognosis
INTRODUCTION
Acute kidney injury (AKI) is a frequent clinical problem in critically ill patients, and the incidence and mortality are still high despite improvements in the knowledge of pathophysiologic mechanisms and in supportive care, including renal replacement therapy (RRT) (1, 2). Serum creatinine-based detection of AKI is thought to hamper timely diagnosis and intervention, because it is a suboptimal marker of kidney injury, with relatively low sensitivity and specificity. There have been intensive investigative efforts to identify an optimal biomarker for sensitive early detection of kidney injury, and several promising serum and urine biomarkers have been identified (3). However, for clinical utility and to replace current use of serum creatinine, these biomarkers need to be extensively validated in a heterogeneous population.
Urinary liver-type fatty acid-binding protein (L-FABP) reflects renal tubular injury and has been shown as a promising biomarker in both chronic kidney disease (CKD) and AKI (4, 5). This study was aimed first to determine the diagnostic value of urinary L-FABP compared to urinary neutrophil gelatinase-associated lipocalin (NGAL), one of the most widely investigated biomarkers of AKI, and secondly, to investigate the ability of urinary L-FABP for predicting prognosis in heterogeneous critically ill patients with or without AKI.
MATERIALS AND METHODS
Patients
This study was a prospective observational study conducted between July 2010 and June 2011. We enrolled adult patients older than 18 yr who were admitted to the medical or surgical intensive care units (ICUs) at the Korea University Medical Center, Seoul, Korea. Patients with end-stage renal disease or kidney transplantation and those with life expectancy of < 48 hr were excluded.
Definitions
AKI was diagnosed by the change of serum creatinine until 5 days of ICU admission according to the Acute Kidney Injury Network (AKIN) criteria (i.e., ≥ 0.3 mg/dL or ≥ 50% increase of serum creatinine from baseline value within 48 hr) (6). The glomerular filtration rate (GFR) was estimated using the Modification of Diet Renal Disease equation, and the baseline serum creatinine was defined as the level at steady state prior to hospitalization. We obtained serum creatinine values from this center or other hospitals or from the routine laboratory examination annually provided by the National Health Service of Korean Medical Insurance System. When this was not available, the lowest level during hospitalization or after discharge was used. CKD was defined as baseline GFR < 60 mL/min/1.73 m2. The presence of liver disease was defined as having known liver cirrhosis or more than 3 times increase of alanine aminotransferase than the upper normal value (40 IU/L) in our laboratory test.
Data collection
The patient demographic data, comorbidities, type of admission, and etiology of AKI were obtained, and the following data were prospectively collected: 6 hr urine output, total fluid balance during 24 and 72 hr, RRT requirement, and other physiologic and clinical variables composing the simplified acute physiology score II (SAPS II) (7). Each patient was followed up to 90 days from the date of ICU admission or until death, whichever came first. The survival was assessed through phone calls when needed.
Measurement of urinary NGAL and L-FABP
Urine samples were collected at the time of ICU admission and centrifuged at 2,500 rpm at 4℃ for 5 min. The supernatants were frozen at -80℃ until the biomarker assay was performed. Urinary NGAL and L-FABP were measured using NGAL ELISA kit (BioPorto, Gentofte, Denmark) and human L-FABP Assay kit (CMIC Co Ltd, Tokyo, Japan), respectively, according to the manufacturer's instructions.
Statistical analysis
Continuous variables were expressed as means with standard deviation (SD) and compared using Student's t-test. When variables were not normally distributed, they were presented as median and interquartile range (IQR) and compared using the Mann-Whitney U test. Categorical variables were compared using chi-square test or Fisher's exact test. The performance of urinary biomarkers in detection of AKI was assessed using receiver operating characteristic (ROC) curve analysis. The comparison between area under the ROC curves (AUC-ROC) was determined using MedCalc program. We identified prognostic factors for 90 day mortality with multivariate analysis in the Cox proportional hazard model by the backward stepwise method and evaluated the performance of predictors by plotting ROC curves. We also depicted Kaplan-Meier curves to evaluate the impact of urinary L-FABP on survival. Log-rank test was used to test differences in survival. All statistical analysis was performed with SPSS version 18, except comparing ROC curves, which used MedCalc program. P value < 0.05 was considered statistically significant.
Ethics statement
The study was approved by the institutional review board of Korea University Anam Hospital (IRB No. 1159), and written informed consent was confirmed by the IRB and obtained from each patient or legal guardian.
RESULTS
Patient Characteristics and outcomes
During one year of the study period, 145 patients (84 male, 58.6%; mean age, 62 yr) were enrolled, and 75 (51.7%) had medical causes of admission. Among them, AKI compromised 54 (37.2%) patients; 37 had established AKI at the time of ICU admission, while the remaining 17 had newly developed AKI within 5 days of ICU admission. The etiologies of AKI were diverse, but sepsis was the leading cause of AKI (26 patients, 48.1%) and others were as follows: ischemic in 9 (16.7%), associated with brain lesion such as intracranial hemorrhage in 8 (14.8%), associated with heart failure in 3 (5.6%), drug intoxication in 1 (1.9%), and multifactorial including postoperative AKI in 7 (12.9%). Patients with both established and newly developed AKI were placed in the AKI group. Regarding the AKIN stage, 28 (51.9%) patients were classified as stage 1, 13 patients as stage 2 and another 13 patients as stage 3. The clinical characteristics of the AKI versus non-AKI group are presented in Table 1. Diabetes and CKD were more prevalent in the AKI group than the non-AKI group (40.7% vs 20.9%, P = 0.013; 22.2% vs 8.8%, P = 0.044, respectively). Patients with AKI had higher SAPS II scores, less 6 hr urine output, and more positive fluid balance with wider range during the first day of ICU admission. Table 2 shows the types of surgery in patients admitted with surgical indications. Intra-abdominal surgery was the most common cause of surgical admissions in patients with AKI. In the AKI group, 9 (17%) patients required RRT, and 34 (62.9%) recovered their renal function at hospital discharge. Overall, 19 (13.1%) and 28 (19.3%) patients showed 30 day and 90 day mortality, respectively. Patients with AKI had significantly worse prognosis as presented in Table 1. Additionally, we also assessed the 90 day mortality only in patients who survived at 30 days of ICU admission to assess whether an episode of AKI lead to additional mortality. There was no significant difference in additional mortality at 90 days in patients with AKI compared to those without AKI (11.9% vs 4.8%, P = 0.162).
Table 1.
Basic characteristics and outcomes
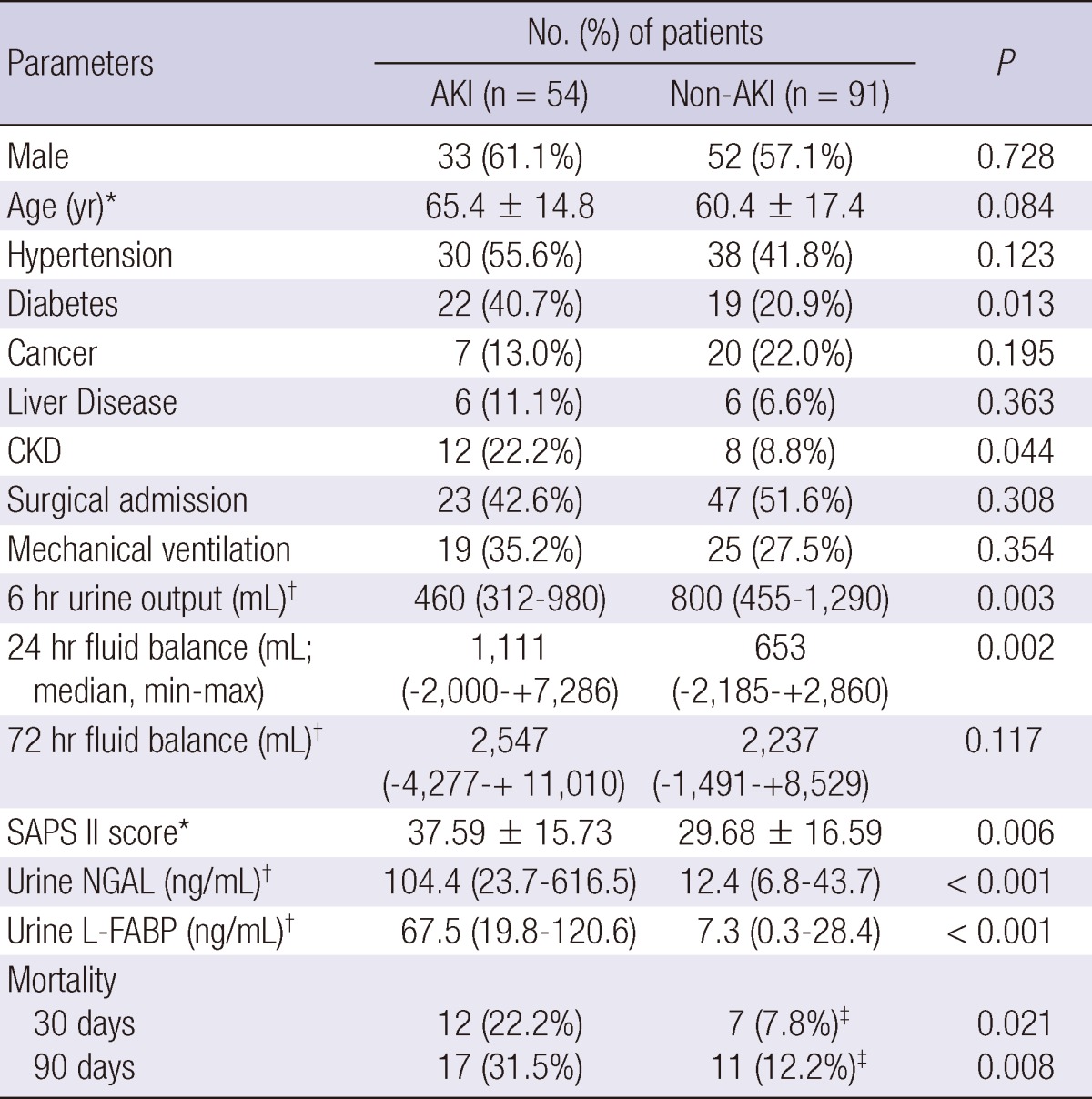
*Mean ± SD; †median (interquartile range); ‡for survival analysis, 90 patients were assessed in the non-AKI group. AKI, acute kidney injury; CKD, chronic kidney disease; SAPS, simplified acute physiology score; NGAL, neutrophil gelatinase-associated lipocalin; L-FABP, liver-type fatty acid binding protein.
Table 2.
The analysis of surgical admissions
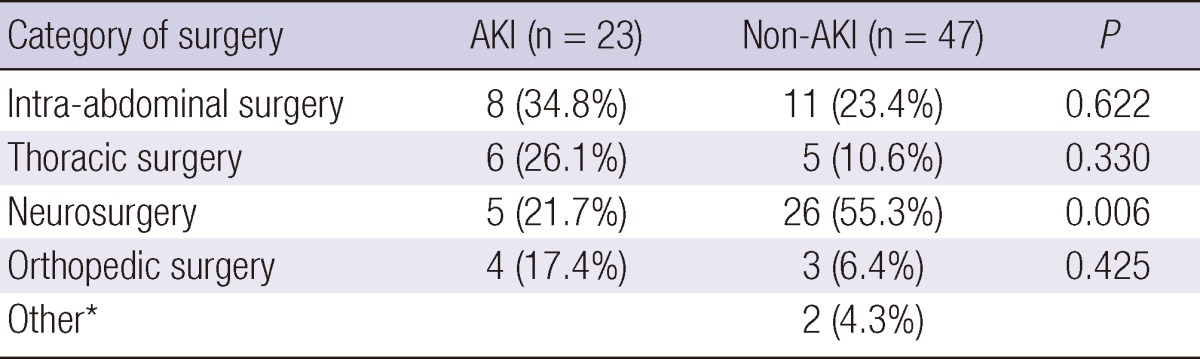
*Two patients in non-AKI group were admitted with intracranial hemorrhage, but did not have an operation and ended up as cadaver donors. AKI, acute kidney injury.
Diagnostic performance of urinary biomarkers for AKI
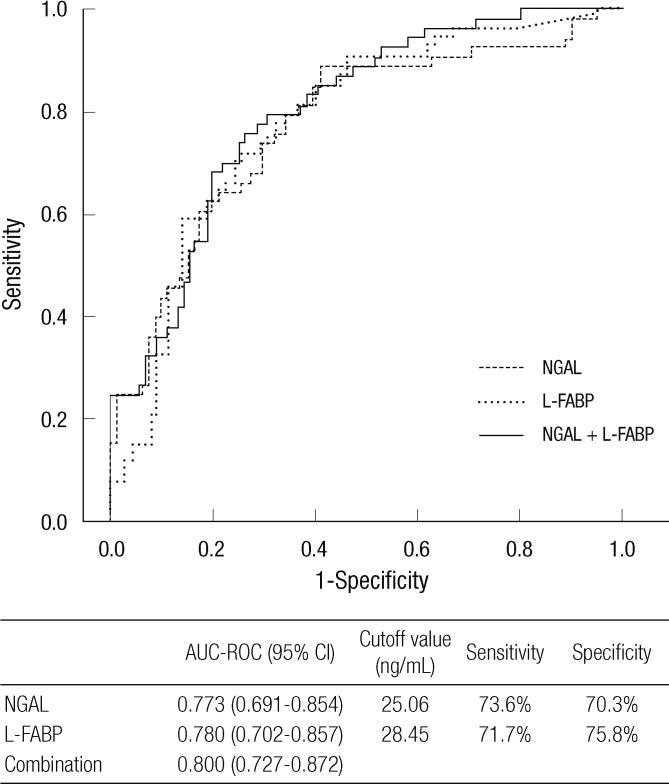
Both urinary NGAL and L-FABP at the time of ICU admission were significantly higher in patients with AKI (Table 1). We evaluated and compared the diagnostic performances of urinary biomarkers for AKI by calculating the area under the ROC curves. Fig. 1 displays the ROC curves of both urinary biomarkers and the combination of the two. This analysis demonstrated that urinary L-FABP could detect AKI a little better than urinary NGAL, with the AUC of 0.780 (95% confidence interval [CI] 0.702-0.857, with a sensitivity of 71.7% and a specificity of 75.8% at a cutoff value of 28.45 ng/mL). The combination of both urinary biomarkers showed slightly greater diagnostic performance than each biomarker; however, there were no significant differences between them.
Fig. 1.
Diagnostic performance for AKI. Receiver operating characteristic curves for the diagnosis of AKI. The area under the ROC curves and the cutoff value of each urinary biomarker are presented in the separate table under the figure.
Predictive factors for 90 day mortality and their prognostic performance
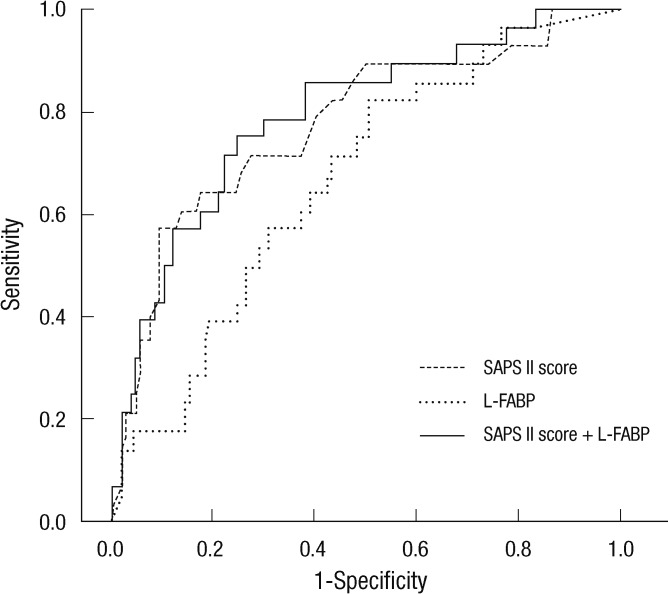
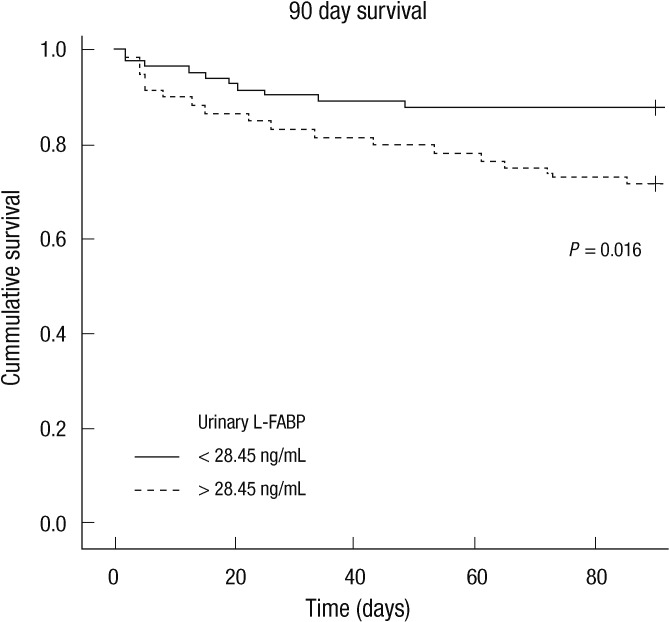
Twenty-eight among 144 critically ill patients died during the 90 days of the follow-up period. Survival data was unavailable for one patient. Nonsurvivors had significantly elevated initial urinary L-FABP and NGAL compared to survivors (Table 3); urinary L-FABP (median, 57.16 vs 10.88 ng/mL, P = 0.004) and urinary NGAL (median, 68.18 vs 18.13 ng/mL, P = 0.010). To determine the predictive value of urinary biomarkers in predicting patient prognosis, we performed multivariate Cox regression analysis. Variables included in the model were tested for multicolinearity and were as follows: age, gender, hypertension, diabetes, cancer, liver disease, CKD, fluid balance for 24 and 72 hr, 6 hr urine output, requirement of mechanical ventilation or RRT, SAPS II score, and urinary L-FABP and NGAL. The presence of CKD was the most powerful predictor for 90 day mortality (Table 4). SAPS II score and urinary L-FABP, but not urinary NGAL, were also found to be independent predictors of 90 day mortality in the heterogeneous ICU population. The AUC-ROC was calculated for SAPS II score (0.778, 95% CI 0.675-0.880) and urinary L-FABP (0.669, 95% CI 0.563-0.775) and depicted in Fig. 2. However, when we performed subgroup analysis including only patients without AKI, the level of urinary L-FABP showed no significant difference between the survivor and the dead patients. And unfortunately, the combination of urinary L-FABP with the SAPS II score did not significantly improve the predictive ability for 90 day mortality (AUC 0.794, 95% CI, 0.699-0.890). In Fig. 3, we also plotted Kaplan-Meier survival curves using a cutoff value of urinary L-FABP for detecting AKI (28.45 ng/mL). Patients with higher urinary L-FABP showed shorter mean overall survival than patients with lower urinary L-FABP (73.1 ± 3.9 days vs 81.3 ± 2.6 days, P = 0.016 by the log-rank test).
Table 3.
Urinary biomarkers and SAPS II score in survivor versus non-survivor
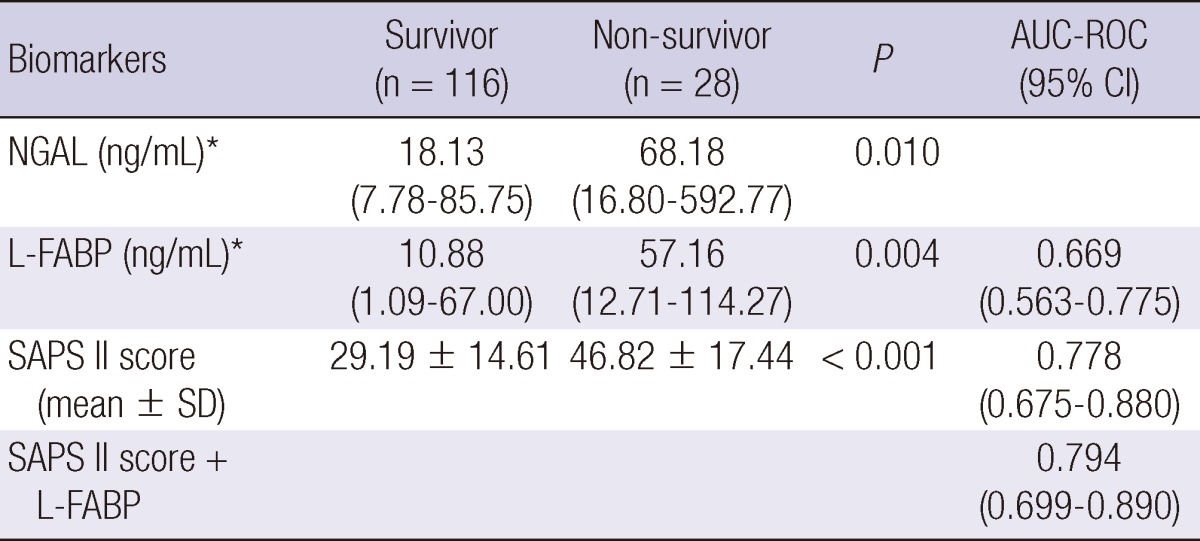
*Median (interquartile range). SAPS, simplified acute physiology score; AUC-ROC, area under curve-receiver operating characteristic; NGAL, neutrophil gelatinase-associated lipocalin; L-FABP, liver-type fatty acid binding protein.
Table 4.
Independent predictors for 90 day mortality
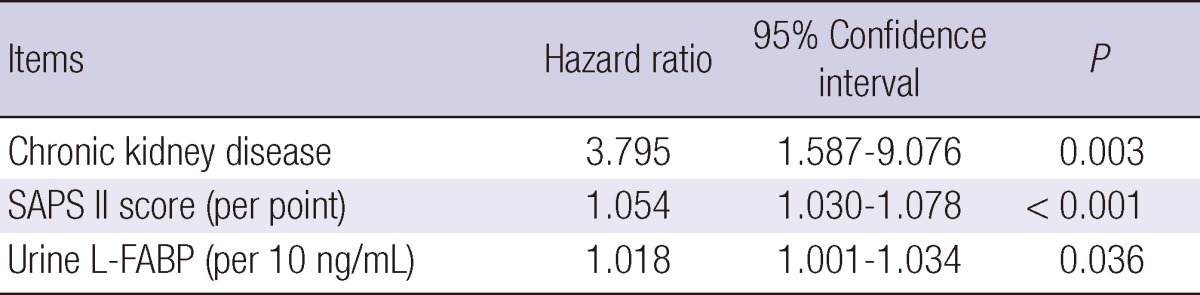
SAPS, simplified acute physiology score; L-FABP, liver-type fatty acid binding protein.
Fig. 2.
Predictive performance for 90 day mortality. Receiver operating characteristic curves of SAPS II score and urinary L-FABP for 90 day mortality. The AUC-ROCs are presented in Table 2.
Fig. 3.
Kaplan-Meier survival curves according to the level of urinary L-FABP. Log-Rank test.
DISCUSSION
Acute kidney injury is a continuing clinical problem, especially in critically ill patients. It occurs in 30%-50% of ICU patients and is associated with substantial mortality, despite advancements in laboratory science and critical care medicine. In current practice, AKI is diagnosed based on changes of serum creatinine concentration, with or without oliguria. However, serum creatinine measurement is relatively insensitive and nonspecific for the detection of early kidney injury, which limits timely diagnosis and intervention, thus ultimately compromising patient outcome. Over the last decade, many efforts have been made to identify an ideal biomarker for detecting early stage of AKI and also to evaluate the prognostic performances of several candidate biomarkers, including kidney injury molecule 1 (KIM-1), N-acetyl-β-D-glucosaminidase (NAG), NGAL, and interleukin-18 (IL-18) (8, 9).
Serum and urine NGAL have been most intensively investigated across a wide range of clinical settings of AKI in pediatric and adult patients (10-12). Haase et al. (13) recently reviewed 19 studies involving 2,538 patients and reported that the NGAL level appeared to serve as a useful diagnostic and prognostic tool of AKI. L-FABP is a newly emerging biomarker that has antioxidant properties, and enhanced expression in proximal tubule cells and subsequent urinary excretion are known to reflect the presence of tubular injury. In fact, Yokoyama et al. (14) reported that the amount of renal expression and urinary excretion of L-FABP were significantly correlated with the severity of tubulointerstitial damage in human L-FABP transgenic mice. Urinary L-FABP has also been validated in multiple clinical situations along with other known biomarkers of AKI (15-17).
Until recently, most attempts to assess the usefulness of novel biomarkers have been performed in homogeneous populations such as patients with cardiac surgery. For the application of these biomarkers in clinical practice, however, the focus of clinicians has now moved to performing studies in heterogeneous populations who were known to have increased risk of AKI. Ferguson et al. (18) performed a cross-sectional study using 92 patients with established AKI and 68 controls composed of four different groups. They found that urinary L-FABP had good performance in detecting AKI and significant predictive value for RRT requirement and for the composite end point of death/RRT, regardless of the etiology of AKI. More recently, Doi and colleagues (19) conducted a prospective observational study evaluating the performance of five urinary biomarkers (L-FABP, NGAL, IL-8, NAG, and albumin) in 339 adult ICU patients. They also demonstrated that urinary L-FABP had better diagnostic ability for the detection and prediction of AKI than other biomarkers, and also observed that L-FABP, along with urinary NGAL, were significantly associated with 14-day in-hospital mortality.
Our study further investigated the diagnostic and prognostic value of urinary L-FABP in heterogeneous ICU patients, performing a prospective observational cohort study with extension of the follow-up period up to 90 days. As far as we know, there have been no studies that evaluate the role of urinary L-FABP in predicting relatively long-term outcome in a heterogeneous ICU population both with and without AKI. Overall, patients with AKI had more severe burden of illness and showed worse outcome during 90 days of follow-up than patients without AKI. Urinary L-FABP and NGAL were significantly higher in the AKI group, which included patients with both established and newly developed AKI. The ability of urine L-FABP to detect AKI was comparable with that of urine NGAL, with AUCs of 0.780 and 0.773, respectively. This result again confirmed previous studies. In this study, urine L-FABP was measured only once at the time of ICU admission, and this should not be able to reflect the time of AKI development. If urine can be collected at the similar time point of AKI development and progression in future studies, the diagnostic ability can be more accurate. Also, the cutoff values were different from study to study depending on the target population. For example, the cutoff value of urinary L-FABP was 486 ng/mg creatinine (Cr) for AKI prediction after cardiac surgery (17), but it was 47.1 ng/mg Cr to identify established AKI from hospitalized patients in a cross-sectional study (18). Herein, we examined the cutoff value for detection of both established and newly developed AKI in a heterogeneous ICU population, and it was 28.45 ng/mL for L-FABP and 25.06 ng/mL for NGAL. More intense studies are needed to determine a reliable cutoff value in clinical practice. In this study, we used the absolute concentration of urinary biomarkers rather than the values that were normalized to urinary creatinine. Although the issue whether absolute concentration or normalized value against urinary creatinine should be used is still controversial, evidences are accumulating that suggest that absolute level of urinary biomarkers can be applicable to clinical evaluation (19-21). It is also noteworthy that we tested whether the combination of both urinary biomarkers improved the detection ability for AKI. We could not observe any significant increase in AUC from combination of these two biomarkers. However, the possible effect of developing panel of biomarkers that can enhance its diagnostic ability should not be underestimated from this result (22).
Thus far, many studies have assessed biomarkers as outcome predictors, adjusting only for age and gender. In the present study, we included various confounders in a multivariate Cox regression model to find independent predictors of 90 day mortality, such as fluid balance, urine output, acute illness score, and underlying diseases. Our study revealed that urinary L-FABP, but not NGAL, was the independent biomarker with prognostic ability in heterogeneous ICU population including both with and without AKI. At a cut-off value of 28.45 ng/mL, Kaplan-Meier curves showed significant differences in survival. CKD had the highest hazard ratio (3.795) among independent outcome predictors, and it reminds us that baseline characteristics of patients should always be concerned even when investigators focus on biomarkers. Recent studies reported that fluid overload was associated with increased mortality and suggested fluid balance as a biomarker for adverse outcome (23-25). In this study, day one and day three fluid balance did not independently predict 90 day mortality, although patients with AKI had less 6 hr urine output and higher positive fluid balance with wider range than the non-AKI group. However, the importance of fluid balance should not be underestimated from this result. On the other hand, using the absolute concentration of urinary L-FABP at the time of admission was again found to be useful in predicting patient prognosis, because it was shown to be independently associated with 90 day mortality, even after adjusting for urine output and fluid balance. We also plotted ROC curves of the SAPS II score, urinary L-FABP, and the combination of the two to find the predictive performance for 90 day mortality. However, the improvement of predictive performance with the combination was not significant in our study. Nevertheless, as mentioned earlier, development of panel assays of outcome predictors are still warranted for early detection and outcome prediction.
Although this prospective study revealed some important findings, there are several limitations. First, this is a single center study with a relatively small sample size. Second, the AKI group in our study included both newly developed AKI and established AKI, and because of the too small sample size, we could not assess the true predictive ability of urinary biomarkers for earlier detection of AKI compared to serum creatinine. Third, as in all other biomarker studies, urinary NGAL and L-FABP were compared to serum creatinine, which is insensitive and nonspecific for kidney injury but defines AKI in clinical practice. Fourth, serial measurement of urinary biomarkers will provide better information for future usage of these markers, although a single urine sample at the time of ICU admission is the easiest method to use in clinical practice.
In conclusion, AKI in ICU patients is still a challenging problem with a high incidence and mortality. Numerous biomarkers have been investigated, because timely diagnosis and intervention in AKI is crucial to improve patient outcome. This study suggests that urinary L-FABP could be an adjunctive and independent biomarker for both the detection of AKI as well as the prediction of prognosis in heterogeneous ICU patients. To validate these results and develop a panel of reliable biomarkers, further studies are required.
ACKNOWLEDGMENTS
The authors have no conflicts of interest to disclose.
References
- 1.Bagshaw SM, George C, Bellomo R. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11:R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoste EA, Kellum JA. Acute kidney injury: epidemiology and diagnostic criteria. Curr Opin Crit Care. 2006;12:531–537. doi: 10.1097/MCC.0b013e3280102af7. [DOI] [PubMed] [Google Scholar]
- 3.Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73:1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- 4.Kamijo A, Sugaya T, Hikawa A, Yamanouchi M, Hirata Y, Ishimitsu T, Numabe A, Takagi M, Hayakawa H, Tabei F, et al. Urinary liver-type fatty acid binding protein as a useful biomarker in chronic kidney disease. Mol Cell Biochem. 2006;284:175–182. doi: 10.1007/s11010-005-9047-9. [DOI] [PubMed] [Google Scholar]
- 5.Matsui K, Kamijo-Ikemori A, Hara M, Sugaya T, Kodama T, Fujitani S, Taira Y, Yasuda T, Kimura K. Clinical significance of tubular and podocyte biomarkers in acute kidney injury. Clin Exp Nephrol. 2011;15:220–225. doi: 10.1007/s10157-010-0384-y. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 8.Lisowska-Myjak B. Serum and urinary biomarkers of acute kidney injury. Blood Purif. 2010;29:357–365. doi: 10.1159/000309421. [DOI] [PubMed] [Google Scholar]
- 9.Kang HK, Kim DK, Lee BH, Om AS, Hong JH, Koh HC, Lee CH, Shin IC, Kang JS. Urinary N-acetyl-β-D-glucosaminidase and malondialdehyde as a markers of renal damage in burned patients. J Korean Med Sci. 2001;16:598–602. doi: 10.3346/jkms.2001.16.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makris K, Markou N, Evodia E, Dimopoulou E, Drakopoulos I, Ntetsika K, Rizos D, Baltopoulos G, Haliassos A. Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med. 2009;47:79–82. doi: 10.1515/CCLM.2009.004. [DOI] [PubMed] [Google Scholar]
- 11.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 12.Prabhu A, Sujatha DI, Ninan B, Vijayalakshmi MA. Neutrophil gelatinase associated lipocalin as a biomarker for acute kidney injury in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Ann Vasc Surg. 2010;24:525–531. doi: 10.1016/j.avsg.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and metaanalysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Yokoyama T, Kamijo-Ikemori A, Sugaya T, Hoshino S, Yasuda T, Kimura K. Urinary excretion of liver type fatty acid binding protein accurately reflects the degree of tubulointerstitial damage. Am J Pathol. 2009;174:2096–2106. doi: 10.2353/ajpath.2009.080780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manabe K, Kamihata H, Motohiro M, Senoo T, Yoshida S, Iwasaka T. Urinary liver-type fatty acid-binding protein level as a predictive biomarker of contrast-induced acute kidney injury. Eur J Clin Invest. 2012;42:557–563. doi: 10.1111/j.1365-2362.2011.02620.x. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Sugaya T, Koide H. Urinary liver-type fatty acid-binding protein in septic shock: effect of polymyxin B-immobilized fiber hemoperfusion. Shock. 2009;31:454–459. doi: 10.1097/SHK.0b013e3181891131. [DOI] [PubMed] [Google Scholar]
- 17.Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, Noiri E, Devarajan P. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73:465–472. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson MA, Vaidya VS, Waikar SS, Collings FB, Sunderland KE, Gioules CJ, Bonventre JV. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2010;77:708–714. doi: 10.1038/ki.2009.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doi K, Negishi K, Ishizu T, Katagiri D, Fujita T, Matsubara T, Yahagi N, Sugaya T, Noiri E. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med. 2011;39:2464–2469. doi: 10.1097/CCM.0b013e318225761a. [DOI] [PubMed] [Google Scholar]
- 20.Yang HN, Boo CS, Kim MG, Jo SK, Cho WY, Kim HK. Urine neutrophil gelatinase-associated lipocalin: an independent predictor of adverse outcomes in acute kidney injury. Am J Nephrol. 2010;31:501–509. doi: 10.1159/000309841. [DOI] [PubMed] [Google Scholar]
- 21.Vaidya VS, Ford GM, Waikar SS, Wang Y, Clement MB, Ramirez V, Glaab WE, Troth SP, Sistare FD, Prozialeck WC, et al. A rapid urine test for early detection of kidney injury. Kidney Int. 2009;76:108–114. doi: 10.1038/ki.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011;23:194–200. doi: 10.1097/MOP.0b013e328343f4dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagshaw SM, Brophy PD, Cruz D, Ronco C. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Crit Care. 2008;12:169. doi: 10.1186/cc6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouchard J, Mehta RL. Fluid accumulation and acute kidney injury: consequence or cause. Curr Opin Crit Care. 2009;15:509–513. doi: 10.1097/MCC.0b013e328332f653. [DOI] [PubMed] [Google Scholar]
- 25.Shum HP, Lee FM, Chan KC, Yan WW. Interaction between fluid balance and disease severity on patient outcome in the critically ill. J Crit Care. 2011;26:613–619. doi: 10.1016/j.jcrc.2011.02.008. [DOI] [PubMed] [Google Scholar]