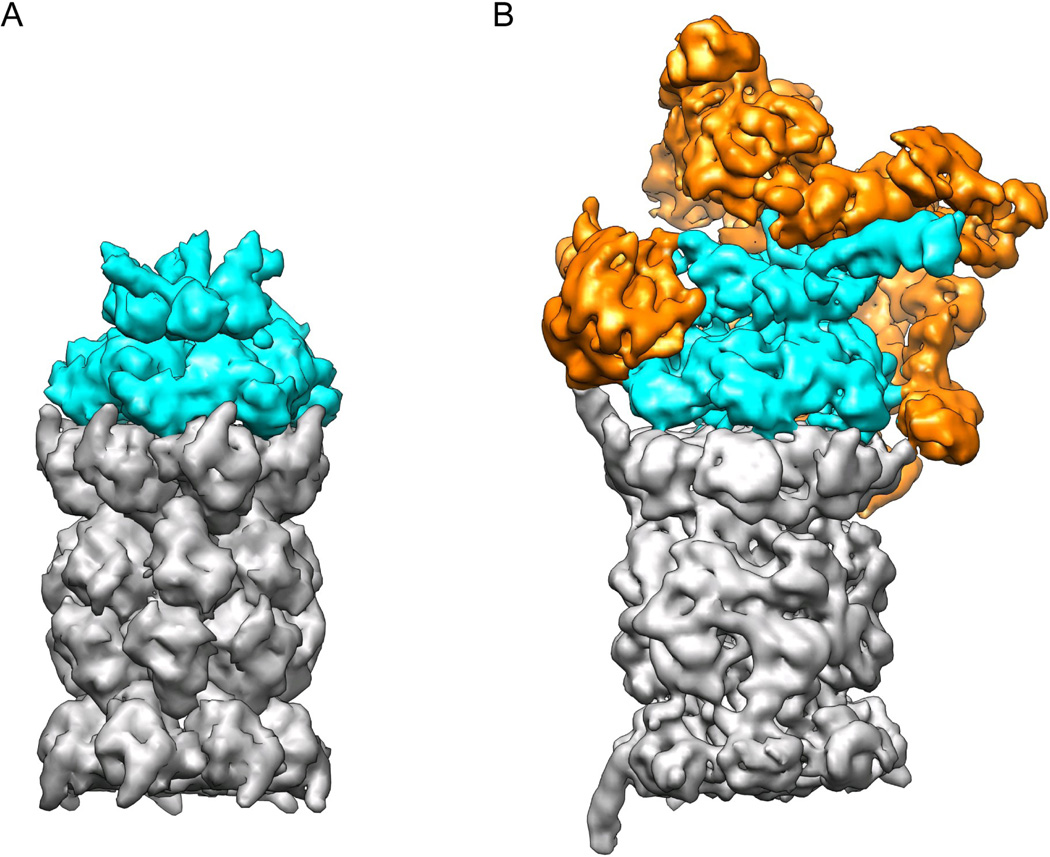
Figure 1. The 26S proteasome is a heteromeric and asymmetric ATP-dependent protease with higher complexity than its prokaryotic ancestors.
A) Model of the homo-hexameric archaeal unfoldase PAN (cyan) bound to the core particle (grey). The separate crystal structures of the N-ring (3H43), the ATPases (3H4M), and core particle (3H4P) 38 were docked together and rendered with a surface resolution of 4 Å to match more closely the EM structure of the 26S proteasome. B) Cryo-EM reconstruction of the 26S proteasome at subnanometer resolution 33. The core particle (grey) and AAA+ unfoldase subunits (cyan) resemble the archaeal PAN-20S protease in size and shape, but bear distinct asymmetries due to their heteromeric ring architectures. The additional structural modules that do not share homology with other compartmental peptidases and have likely been added to accommodate ubiquitin signaling are shown in orange. They include the lid sub-complex, the torroidal subunits Rpn1 and Rpn2, and the ubiquitin receptors.