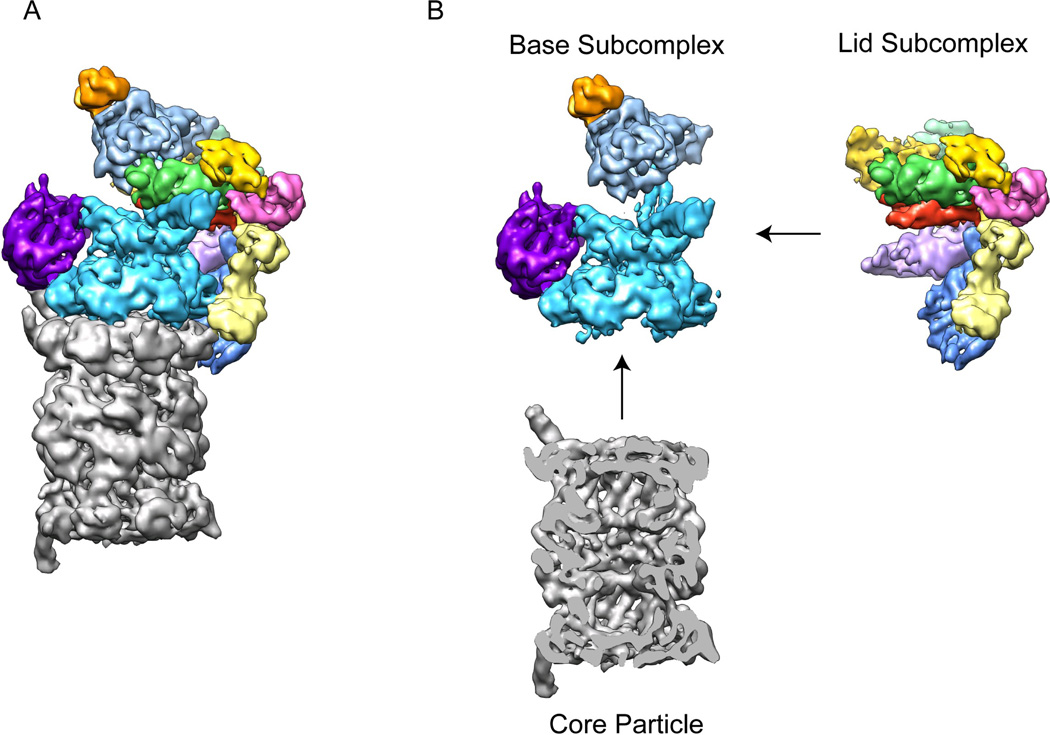
Figure 2. The 26S proteasome can be separated into three stably associated subcomplexes.
A) Cryo-EM reconstruction of the eukaryotic proteasome holoenzyme with the core particle in grey and the regulatory particle multicolored 33. B) Individual sub-complexes of the 26S proteasome. The regulatory particle can be separated into two stable sub-complexes, the lid and the base. The base consists of the AAA+ ATPase subunits Rpt1-6, (cyan), two large torroidal subunits, Rpn1 (purple) and Rpn2 (light blue), and the ubiquitin receptor Rpn13 (orange). The lid contains six PCI-domain subunits (multicolored) along with the essential DUB Rpn11 (green) and the ubiquitin receptor Rpn10 (yellow). A central cross-section of the core particle (grey) is shown to allow visualization of the barrel-shaped central cavity.