Abstract
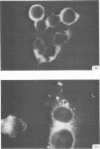
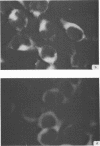
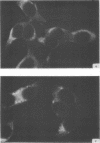
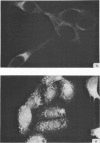
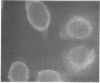
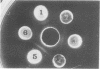
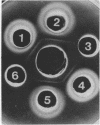
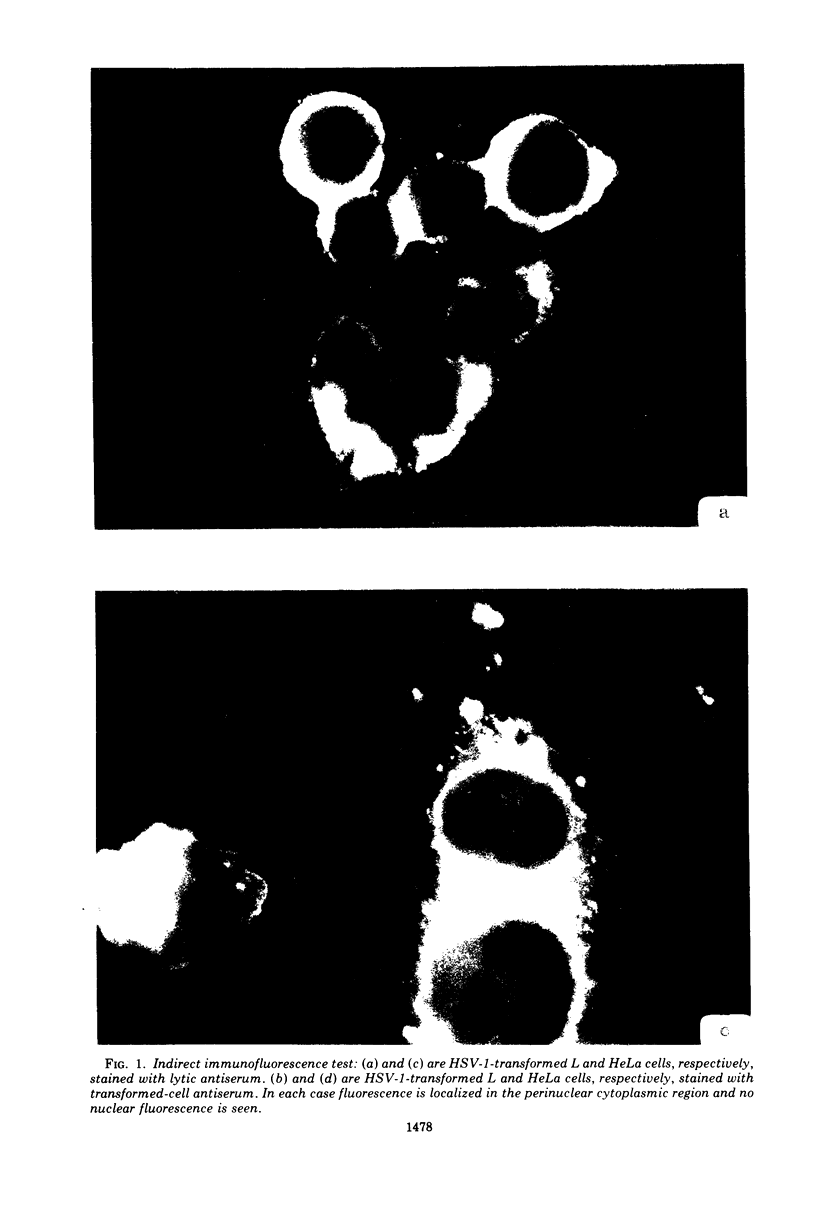
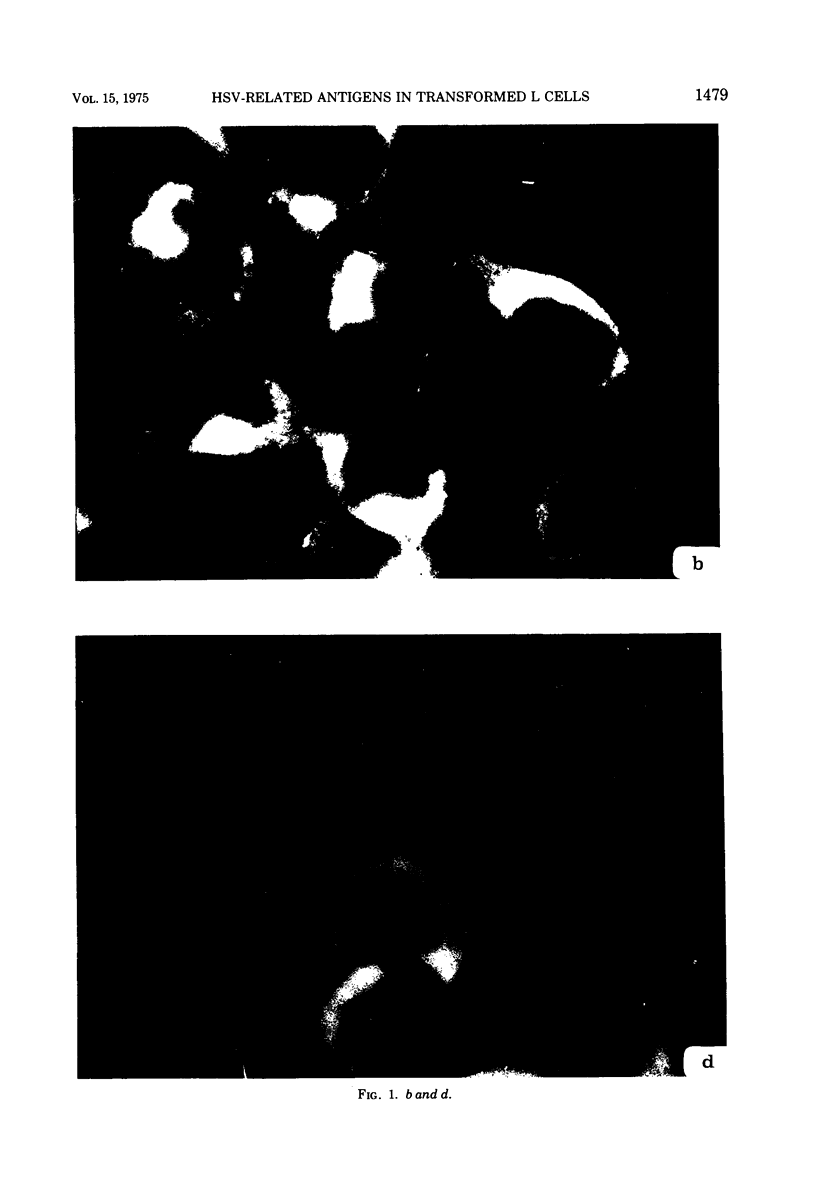
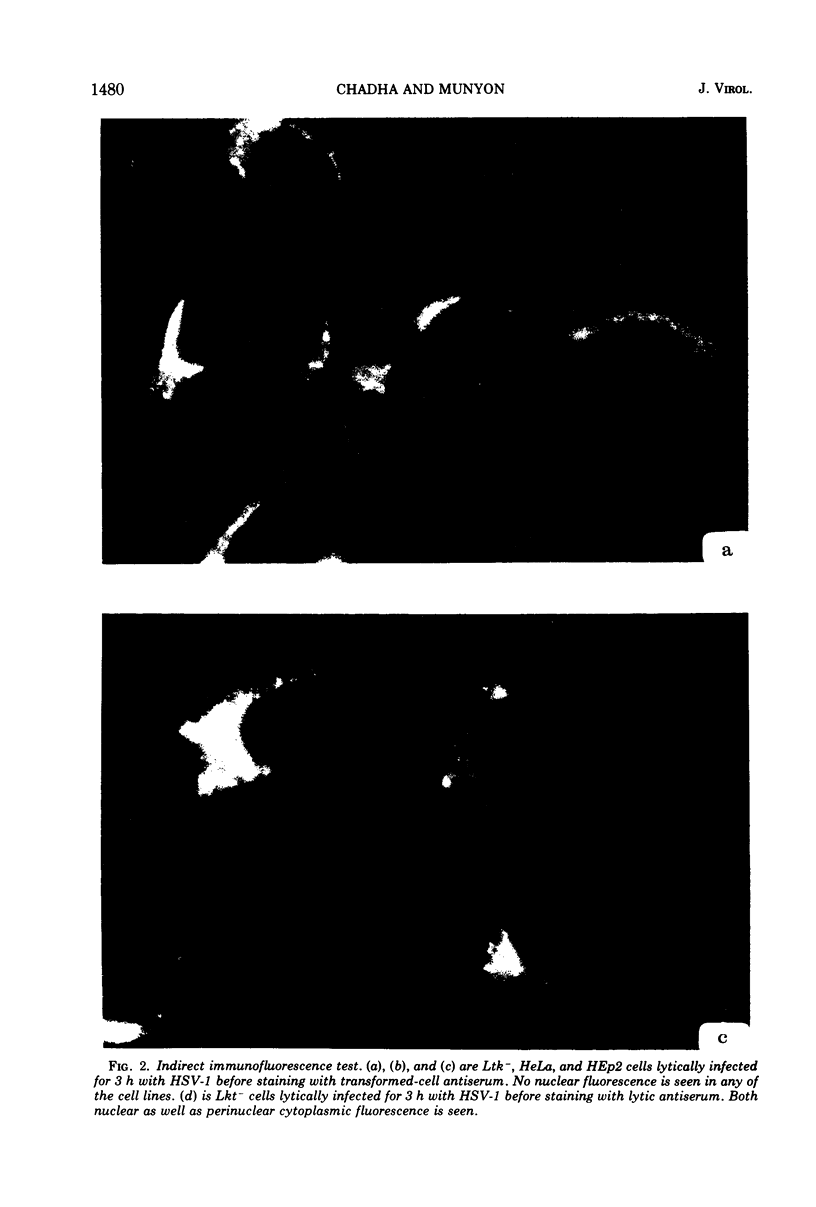
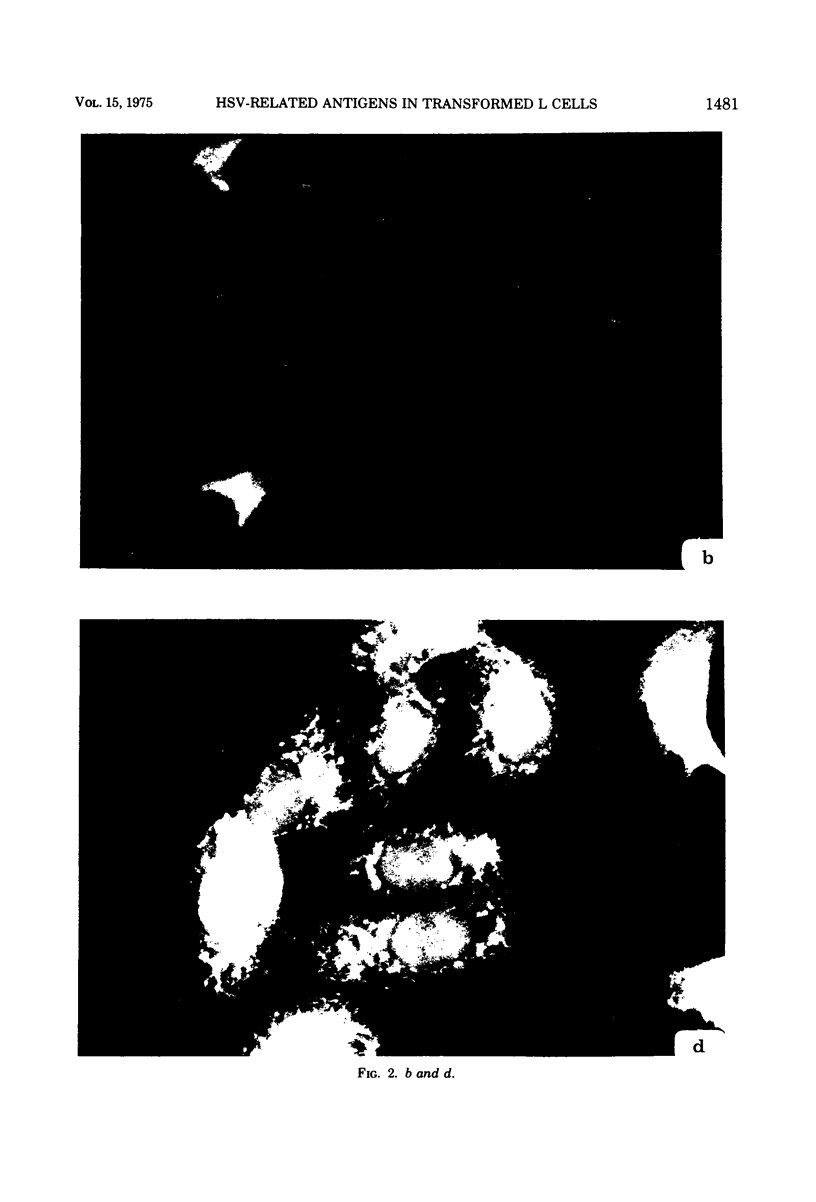
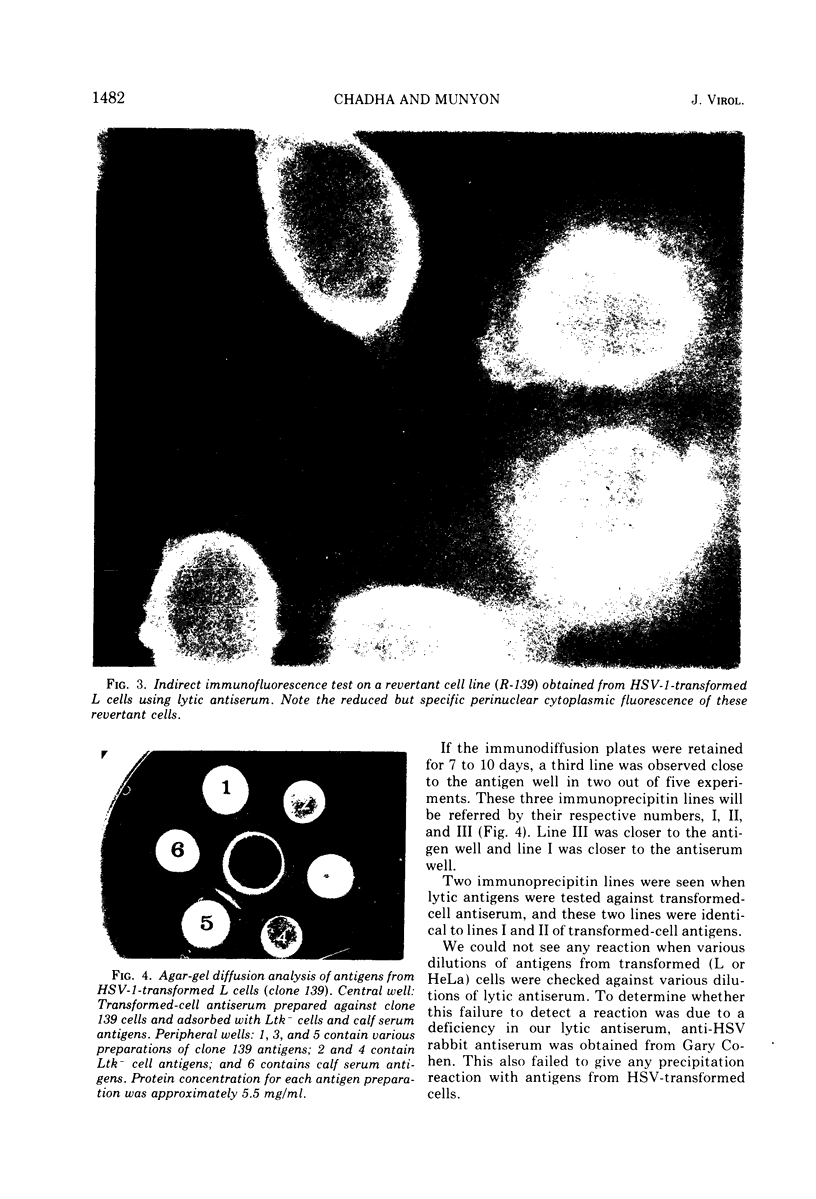
Antiserum prepared against herpes simplex virus type 1 (HSV-1)-infected L cells, i.e., lytic antiserum, was shown by an indirect immunofluorescence test to stain 90 percent of HSV-transformed L or HeLa cells. Immunofluorescence in these cells was always most intense in the perinuclear cytoplasmic region. Similar results were obtained with antiserum prepared against HSV-transformed L cells. These data indicate that HSV-transformed cells (both L and HeLa) express HSV-related antigens. Antiserum prepared against HSV-1-transformed L cells, i.e., transformed-cell antiserum, was found to agglutinate purified HSV type 1 virions but failed to neutralize infectivity. This suggests that HSV-1 structural antigens are expressed in HSV-1-transformed L cells. Immunodiffusion studies showed that at least two HSV-related antigens could be demonstrated with antigens from HSV-1-transformed L cells and transformed-cell antiserum. These two antigens were shown to be present in all clonal lines of HSV-1-transformed cells examined, six L cell lines and one HeLa cell line. Therefore, we conclude that transformation of cells by HSV-1, which is known to be associated with acquisition of viral thymidine kinase, must also be associated with the presence of these two antigens. We performed experiments showing that there are species of HSV-related antibody in HSV-transformed cell antiserum that could not be absorbed out with antigens from HSV-infected L cells. Antibodies present in lytic antiserum were completely removed by antigen preparations from cells lytically infected with HSV-1. Also, lytic antiserum failed to block HSV-related staining of transformed L cells in a direct immunofluorescence test. These results are compatible with one of two notions: either (i) certain genes are expressed during transformation that are not expressed during lytic infection, or (ii) these genes are expressed to a much more reduced extent during lytic infection than in transformed cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida J. D., Waterson A. P. The morphology of virus-antibody interaction. Adv Virus Res. 1969;15:307–338. doi: 10.1016/S0065-3527(08)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe W. K., Notkins A. L. Neutralization of an infectious herpes simplex virus-antibody complex by anti-gamma-globulin. Proc Natl Acad Sci U S A. 1966 Aug;56(2):447–451. doi: 10.1073/pnas.56.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUMSTARK J. S., LAFFIN R. J., BARDAWIL W. A. A PREPARATIVE METHOD FOR THE SEPARATION OF 7S GAMMA GLOBULIN FROM HUMAN SERUM. Arch Biochem Biophys. 1964 Dec;108:514–522. doi: 10.1016/0003-9861(64)90436-9. [DOI] [PubMed] [Google Scholar]
- BLACK P. H., ROWE W. P., TURNER H. C., HUEBNER R. J. A SPECIFIC COMPLEMENT-FIXING ANTIGEN PRESENT IN SV40 TUMOR AND TRANSFORMED CELLS. Proc Natl Acad Sci U S A. 1963 Dec;50:1148–1156. doi: 10.1073/pnas.50.6.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. L., Adelstein S. J., Oxman M. N. Herpes simplex virus as a source of thymidine kinase for thymidine kinase-deficient mouse cells: suppression and reactivation of the viral enzyme. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1912–1916. doi: 10.1073/pnas.70.7.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. B., Munyon W., Buchsbaum R., Chawda R. Virus type-specific thymidine kinase in cells biochemically transformed by herpes simplex virus types 1 and 2. J Virol. 1974 Jan;13(1):140–145. doi: 10.1128/jvi.13.1.140-145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol. 1971 Sep 8;233(36):48–50. doi: 10.1038/newbio233048a0. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster embryo cells after exposure to inactivated herpes simplex virus type 1. J Virol. 1973 Aug;12(2):209–217. doi: 10.1128/jvi.12.2.209-217.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Kaplan A. S. Site of protein synthesis in cells infected with pseudorabies virus. Virology. 1967 May;32(1):60–68. doi: 10.1016/0042-6822(67)90252-8. [DOI] [PubMed] [Google Scholar]
- Garfinkle B., McAuslan B. R. Transformation of cultured mammalian cells by viable herpes simplex virus subtypes 1 and 2. Proc Natl Acad Sci U S A. 1974 Jan;71(1):220–224. doi: 10.1073/pnas.71.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABEL K. SPECIFIC COMPLEMENT-FIXING ANTIGENS IN POLYOMA TUMORS AND TRANSFORMED CELLS. Virology. 1965 Jan;25:55–61. doi: 10.1016/0042-6822(65)90251-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCKINNEY R. M., SPILLANE J. T., PEARCE G. W. FACTORS AFFECTING THE RATE OF REACTION OF FLUORESCEIN ISOTHIOCYANATE WITH SERUM PROTEINS. J Immunol. 1964 Aug;93:232–242. [PubMed] [Google Scholar]
- Munyon W., Buchsbaum R., Paoletti E., Mann J., Kraiselburd E., Davis D. Electrophoresis of thymidine kinase activity synthesized by cells transformed by herpes simplex virus. Virology. 1972 Sep;49(3):683–689. doi: 10.1016/0042-6822(72)90525-9. [DOI] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. Polysomes and protein synthesis in cells infected with a DNA virus. Science. 1966 Jul 1;153(3731):76–78. doi: 10.1126/science.153.3731.76. [DOI] [PubMed] [Google Scholar]
- WELLER T. H., COONS A. H. Fluorescent antibody studies with agents of varicella and herpes zoster propagated in vitro. Proc Soc Exp Biol Med. 1954 Aug-Sep;86(4):789–794. doi: 10.3181/00379727-86-21235. [DOI] [PubMed] [Google Scholar]