Figure 2.
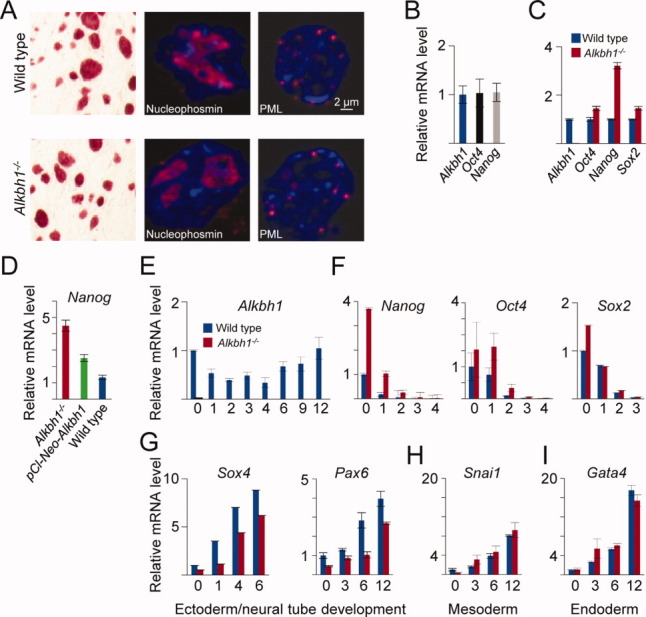
Differentiation is delayed in Alkbh1−/− embryonic stem cells (ESCs). (A): Alkaline phosphatase (AP) staining of wild-type (WT) and Alkbh1−/− mouse ESCs (mESCs). Alkbh1−/− mESCs express markers of pluripotency. (B): The measurement of Alkbh1 expression (blue bar) in undifferentiated mESCs showed expression at the same level as the core pluripotency transcription factors (represented by Oct4 and Nanog). (C): Quantitation of the master regulators of pluripotency revealed upregulation of genes encoding all three transcription factors (Oct4, Nanog, and Sox2) in undifferentiated Alkbh1−/− mESCs. The Nanog gene displayed the greatest (more than fourfold) increase in expression. (D): Quantitative real-time PCR (qRT-PCR) analysis showed that expression of ALKBH1 in Alkbh1−/− mESCs (pCl-Neo-Alkbh1) reduced Nanog expression, albeit not to levels as low as those found in WT mESCs. (E): The qRT-PCR analysis of WT mESCs that were forced to differentiate along an ectodermal path (i.e., by incubation in media without leukemia inhibitory factor and supplemented with 1 μM all-trans retinoic acid) revealed that Alkbh1 was downregulated during the first 4 days of differentiation, before it was upregulated. (F): As expected, levels of expression of the pluripotency markers Oct4, Sox2, and Nanog decreased, albeit at a slower rate in Alkbh1−/− mESCs than in WT mESCs, suggesting delayed differentiation of Alkbh1−/− mESCs. (G): The impeded differentiation of Alkbh1−/− mESCs was confirmed by demonstrating the induction of Sox4 and Pax6 at a later time in the Alkbh1−/− mESCs than in WT mESCs. (H): Expression of the mesodermal marker Snai1 was identical in Alkbh1−/− mESCs and WT mESCs. (I): Expression of the endodermal marker Gata4 was identical in Alkbh1−/− mESCs and WT mESCs. Vertical lines represent the 1 ± SEM. Abbreviations: ALKBH1, AlkB homolog 1; PML, promyelocytic leukemia protein.
