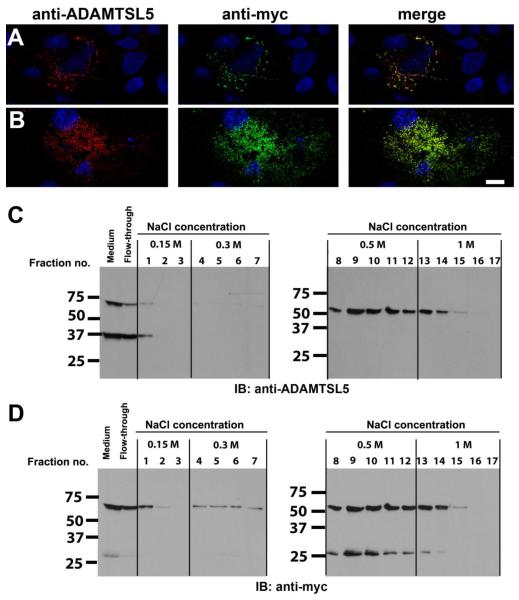
Figure 4. ADAMTSL5 is located at the cell-surface and in the ECM of transiently transfected COS-1 cells and binds heparin.
A,B. Staining of non-permeabilized cells with anti-myc and anti-ADAMTSL5 antibodies gave identical staining patterns. Cos-1 cells were transiently transfected with myc-tagged hADAMTSL5-1 and were co-stained with rabbit anti-ADAMTSL5 (red) and anti-myc (green) antibody with the non-permeabilized staining technique. Nuclei were stained with DAPI and appear blue. Representative confocal images showing cell surface association (A, optical plane through mid-cell region) and ECM association (B, subcellular optical plane) are shown (see supplemental movie). Note that the staining pattern obtained with the two antibodies coincides, indicating that the signal is derived from full-length ADAMTSL5. Scale bar=10 μm. C,D. Heparin-agarose beads were incubated with conditioned medium from HEK293 cells stably transfected with hADAMTSL5-1. After incubation, the beads were washed and eluted with increasing salt concentrations as indicated. ADAMTSL5 was detected in the fractions by western blot using the rabbit anti-ADAMTSL5 antibody (C). After stripping, the blots were reprobed with a myc-antibody (D). Full-length ADAMTSL5 was eluted with 0.5–1 M NaCl, indicating strong binding to heparin. Furthermore, the C-terminal NTR-module containing 27 kDa fragment, which is myc-tagged, bound to the heparin-agarose with similar affinity as the full-length protein, whereas the N-terminal cleavage product of ADAMTSL5 (37 kDa fragment in G) was not retained by the heparin column (compare C and D). This suggests that the NTR-module mediated heparin binding.