Abstract
Background
Use of rectal MRI evaluation of patients with rectal cancer for primary tumor staging and for identification for poor prognostic features is increasing. MR imaging permits precise delineation of tumor anatomy and assessment of mesorectal tumor penetration and radial margin risk.
Objective
To evaluate the ability of pre-treatment rectal MRI to classify tumor response to neoadjuvant chemoradiation.
Design
Retrospective, consecutive cohort study, central review.
Setting
Tertiary academic hospital.
Patients
62 consecutive patients with locally advanced (stage cII-cIII)rectal cancer who underwent rectal cancer protocol high resolution MRI prior to surgery(12/09-3/11).
Main Outcome Measures
Probability of good (ypT0-2N0) vs. poor (≥ypT3N0) response as a function of mesorectal tumor depth, lymph node status, extramural vascular invasion, and grade assessed by uni- and multi-variate logistic regression.
Results
Tumor response was good in 25, 40.3% and poor in 37, 59.7%.Median interval from MRI to OP was 7.9weeks (IQR: 7.0–9.0). MRI tumor depth was <1 mm in 10 (16.9%), 1–5 mm in 30 (50.8%), and >5 mm in 21(33.9%). LN status was positive in 40 (61.5%) and vascular invasion was present in 16 (25.8%). Tumor response was associated with MRI tumor depth (P=0.001), MRI lymph nodes status (P=<0.001)and vascular invasion (P=0.009). Multivariate regression indicated >5mm MRI tumor depth (OR=0.08, 95% CI=0.01–0.93, p=0.04) and MRI LN positivity (OR=0.12, 95% CI=0.03–0.53, p=0.005) were less likely to achieve a good response to neoadjuvant chemoradiotherapy.
Limitations
Uncertain generalizability in centers with limited experience with MRI staging for rectal cancer.
Conclusion
MRI assessment of tumor depth and lymph node status in rectal cancer is associated to tumor response to neoadjuvant chemoradiotherapy. These factors should therefore be considered for stratification of patients for novel treatment strategies reliant on pathologic response to treatment or for the selection of poor-risk patients for intensified treatment regimens.
Keywords: Rectal Cancer, Staging, Magnetic Resonance Imaging, Neoadjuvant Therapy
INTRODUCTION
Multidisciplinary treatment of locally advanced rectal cancer includes preoperative radiotherapy followed by radical rectal resection1. In the U.S. long-course radiotherapy with concurrent chemotherapy is a treatment standard that is associated with improved local control and significant effects of tumor response and downstaging. Following neoadjuvant chemoradiotherapy, up to 20% of patients will have a complete pathologic response and this feature has been associated with a very good prognosis following rectal resection2–4. In other patients a significant tumor burden will remain and these patients will have a higher risk for local and distant treatment failure. Based on these results, there has recently been significant interest in the identification of patients with pathologic complete response for consideration of organ-preserving non-surgical treatment strategies5, 6. In addition, patients with poor risk rectal cancers may benefit from intensified strategies to improve resectability and treatment completion7, 8. However currently it is not possible to accurately stratify patients to these low- and high-risk groups until after neoadjuvant treatment, surgical resection and pathologic evaluation.
High-resolution magnetic resonance imaging has become an important component of rectal cancer staging and multidisciplinary treatment planning, replacing other primary tumor staging modalities in many centers. Advantages of MRI imaging include the ability to establish 3-dimensional relationships between the tumor, rectum, mesorectum, and surrounding structures. Furthermore the depth of mesorectal penetration in the orthogonal plane and the potential for involvement of the mesorectal fascial envelope (radial margin) may be assessed. Tumor involvement of mesorectal or pelvic lymph nodes or the presence of vascular invasion have also been identified with increasing accuracy9, 10. Pre-treatment MRI tumor characteristics havetherefore been used in an effort to classify rectal cancer patients into good and poor prognosis groups. These classifications are primarily based on the anatomic relationships of the tumor to a threatened radial margin, T4 involvement, or the presence of N2 disease and have been used to stratify patients to receive either short-course (5x5) radiotherapy versus long course (45–50.4Gy) chemoradiotherapy but have not been examined as potential treatment response classifiers. There has been growing interest in the use of post-chemoradiation treatment diffusion-weighted MRI imaging to predict tumor response; however this approach is limited by insufficient accuracy for the identification of complete responders and the requirement of post-treatment imaging to make the assessment.
Therefore, we conducted this study to evaluate the ability of tumor characteristics on pre-treatment high-resolution rectal MRI to classify tumor response to neoadjuvant chemoradiation.
PATIENTS and METHODS
Patient Identification
A consecutive cohort of patients with MRI-staged locally advanced (cT3-4 or cN+) rectal cancer treated with preoperative chemoradiotherapy followed by TME surgery at the University of Texas MD Anderson Cancer Center between September, 2009 and March, 2011was identified from our colorectal cancer database and their records were retrospectively reviewed. Primary tumor evaluation included digital rectal examination, proctoscopy, and staging with high-resolution dedicated rectal MRI prior to initiation of long-course neoadjuvant chemoradiation therapy. Patients were excluded if they had concurrent distant metastasis at diagnosis, received short-course (5x5 Gy) radiotherapy, or if the interval from the completion of radiation to surgery was more than 16 weeks. The study was approved by the University of Texas M. D. Anderson Institutional Review Board.
Imaging Technique and Evaluation
All patients underwent high-resolution magnetic resonance imaging of the pelvis for primary rectal cancer staging and computed tomography of the chest and abdomen to exclude distant metastases. MRI was performed on both 1.5T and 3T systems using a cardiac phased-array coil with the patient in supine position. After initial scout imaging, high-resolution sagittal images were obtained to identify the tumor within the rectum. A survey of the pelvis using axial thick slice 2D T2 fast recovery fast spin echo (FRFSE) was completed and additional high-resolution sequences including oblique axial perpendicular to tumor and coronal 2D T2 FRFSE images were acquired. Finally oblique, thin slice 3D T2W cube images were also obtained. Intravenous contrast enhancement was not utilized. MRI characteristics assessed included depth of penetration into the mesorectum, relationship to the mesorectal fascial envelope (circumferential margin), status of the regional lymph nodes(involved, indeterminate or negative), presence of extramural vascular invasion, and maximum tumor depth into the mesorectum as previously described11, 12. Specifically, lymph nodes were classified as malignant if they exhibited irregular borders, mixed signal intensity or both. Smooth bordered lymph nodes with homogeneous signal intensity were classified as indeterminate. If no lymph nodes were seen or small smooth homogenous lymph nodes with fatty hila were seen, they were classified as negative. All images were independently reviewed by two dedicated gastrointestinal radiologists who were blinded to the histopathologic outcomes.
Treatment
The neoadjuvant chemoradiation regimen consisted of pelvic external beam radiation, 45 Gy in 25 fractions over 5 weeks followed in most cases (n=53, 87%) by a boost to the tumor of 5.4 Gy in five fractions, delivered as a second daily fraction in the last week of treatment, taking the cumulative dose to 50.4 Gy. In 7 (11.3%) patients with inguinal or lateral pelvic lymphadenopathy, or with adjacent organ involvement, the boost was 15.2–18 Gy using an intensity-modulated radiation therapy technique. Radiotherapy was delivered using a 3 or 4 field technique by 18-MV photons with customized blocking and in the prone position. Concurrent chemotherapy consisted of capecitabine, 1500 mg twice daily on days of radiation therapy only. In some cases, protocol-based concurrent chemotherapy included the addition of bevacizumab and erlotinib (n=6) or cucurmin (n=5).
The surgical resection plan was determined based on the findings on pre-treatment MRI and adhered to the principles of total mesorectal excision with en bloc resection of any adjacent involved structures and wide resection of the pelvic floor in cases of abdominoperineal resections. Involved lateral lymph nodes were surgically addressed.
Pathologic Assessment
Standard pathologic tumor staging of the resected specimen was performed in accordance with the guide lines of the American Joint Committee on Cancer. Resection specimens were oriented immediately after resection by the surgeon and pathologist and the radial margins of resection were marked. The primary tumor was entirely embedded in paraffin and serial sections were histologically evaluated using standard hemotoxylin and eosin staining. The mesorectum was manually dissected for lymph nodes which were examined with 1–3 separate sections per node. Complete response was defined as absence of viable adenocarcinoma cells in the surgical specimen (ypT0N0).Intermediate response was defined ypT1–2 without lymph node metastases, and poor response was defined as ypT3–4 or if lymph node metastases were identified.
Statistical Analysis
Patient, tumor, and MRI characteristics were evaluated using descriptive statistics. The MRI imaging characteristics were defined as categorical variables. Non-parametric data was assessed as median and interquartile range (IQR). Categorization of variables with continuous measurements (e.g. depth of penetration into the mesorectum) was performed prior to analysis. Logistic regression analyses were performed to examine univariate associations with pathologic response. Pathologic response to treatment was defined as “good” if the final pathology stage was ypT0-2N0 or “poor” if it was ypT3-4 or ypN+. The complete and intermediate response categories were grouped as “good” as they have been associated with more favorable prognosis to yield a binary response classification. A multivariate logistic regression model was then constructed using stepwise forward selection using the terms identified on univariate analysis. We tested for interactions between the independent variables with logistic regression and in cases of collinearity, excluded the redundant terms in the final model. Model discrimination was evaluated by generating a receiver operator curve and determining the concordance index.
RESULTS
Patients and Treatment
A total of 62 patients met eligibility criteria and were analyzed. Median age at diagnosis was 55.5 (IQR, 47 to 62) years. Baseline patient and treatment characteristics are summarized in table 1. Most patients had distal tumors with a median distance of 6 cm from the anal verge (IQR 4–9 cm). All patients underwent preoperative long-course chemoradiation therapy followed by total or tumor specific mesorectal excision with en bloc resection of involved adjacent structures at a median of 8 weeks following the last dose of radiotherapy. Surgical resection was performed at a median 7.9 (IQR 7–9) weeks after the last fraction of radiotherapy. Operations performed were LAR in 15 (24.2%), proctectomy with coloanal reconstruction in 29 (46.8%), abdominoperineal resection in 12 (29.4%) and multivisceral resection in 6 (9.7%).
Table 1.
Patient and Treatment Characteristics
| Variable | N (%) |
|---|---|
| Age (years [IQR]) | 55.5 [47–62] |
| Sex | |
| Female | 30 (48) |
| Male | 32 (52) |
| dAV | |
| >10 cm | 10 (16.3) |
| 5–10 cm | 24 (38.7) |
| ≤ 5 cm | 28 (45.3) |
| Tumor differentiation | |
| Well-moderate | 48 (77.4) |
| Poor-undifferentiated | 14 (22.6) |
| Operation | |
| LAR | 15 (24.2) |
| uLAR/CAA | 29 (46.8) |
| APR | 12 (29.5) |
| Multivisceral | 6 (9.7) |
| CXRT Interval (weeks [IQR]) | 8 [7–9] |
assessed after neoadjuvant therapy
MRI Evaluation
Pre-treatment tumor stage and MRI characteristics are shown in table 2.The majority of tumors had 1–5 mm of penetration into the mesorectum, however 34% (n=21) of the patients had >5 mm penetration.T2 tumors were defined as having 0 mm penetration into the mesorectum.The distance from the primary tumor or involved mesorectal lymphadenopathy to the mesorectal fascia or peritoneal surface in the case of more proximal tumors was evaluated with a median distance of 4 mm [IQR 0–12 mm]. Based on pre-treatment MRI, the resection margins was felt to be at risk in 24 (38.7%) of patients by either primary tumor extension to the mesorectal fascia or by metastatic lymph nodes. Lymph node metastases were assessed to be present in almost two-thirds of the patients (n=40, 61.5%) and extramural vascular invasion was identified in 16 (25.8%) patients. (Figures 1 & 2).
Table 2.
Pre-treatment MRI stage and tumor characteristics
| Variable | N (%) |
|---|---|
| mr T Stage | |
| T2 | 9 (14.5) |
| T3a (<1 mm) | 2 (3.2) |
| T3b (1–5 mm) | 29(46.8) |
| T3c (>5–15 mm) | 10(16.1) |
| T3d (>15 mm) | 5 (8.1) |
| T4 | 7 (11.3) |
| mr N Stage | |
| N0 | 3 (4.8) |
| N indeterminate | 21 (33.9) |
| N+ | 43 (61.3) |
| CRM threatened (≤1 mm) | 24 (38.7) |
| mm to mesorectal fascia [IQR] | 4 [0–11.5] |
| Vascular invasion | |
| Yes | 16 (25.8) |
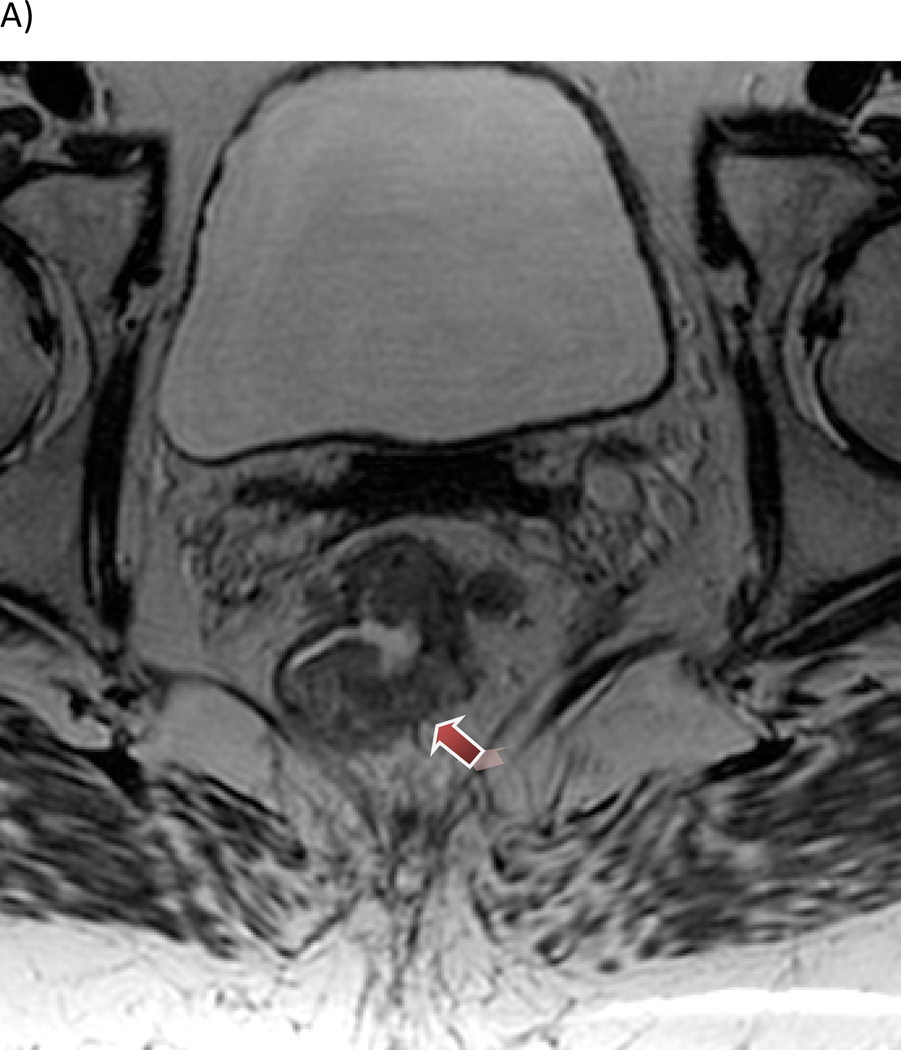
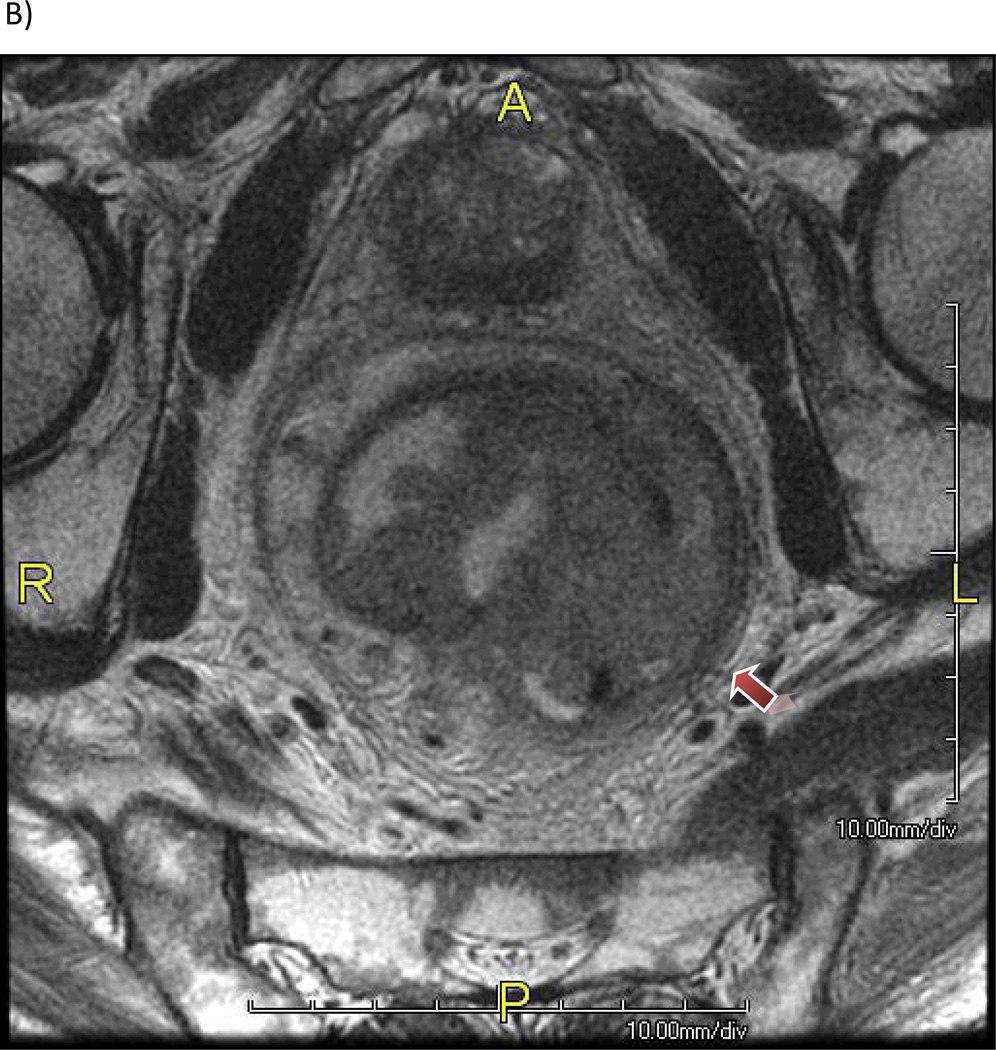
Figure 1.
Patient with a mrT3 tumor with (A) 5 mm extension beyond the outer border of the muscularis propria layer (2D T2W cube) and (B) extensive involvement of the mesorectal fascia.
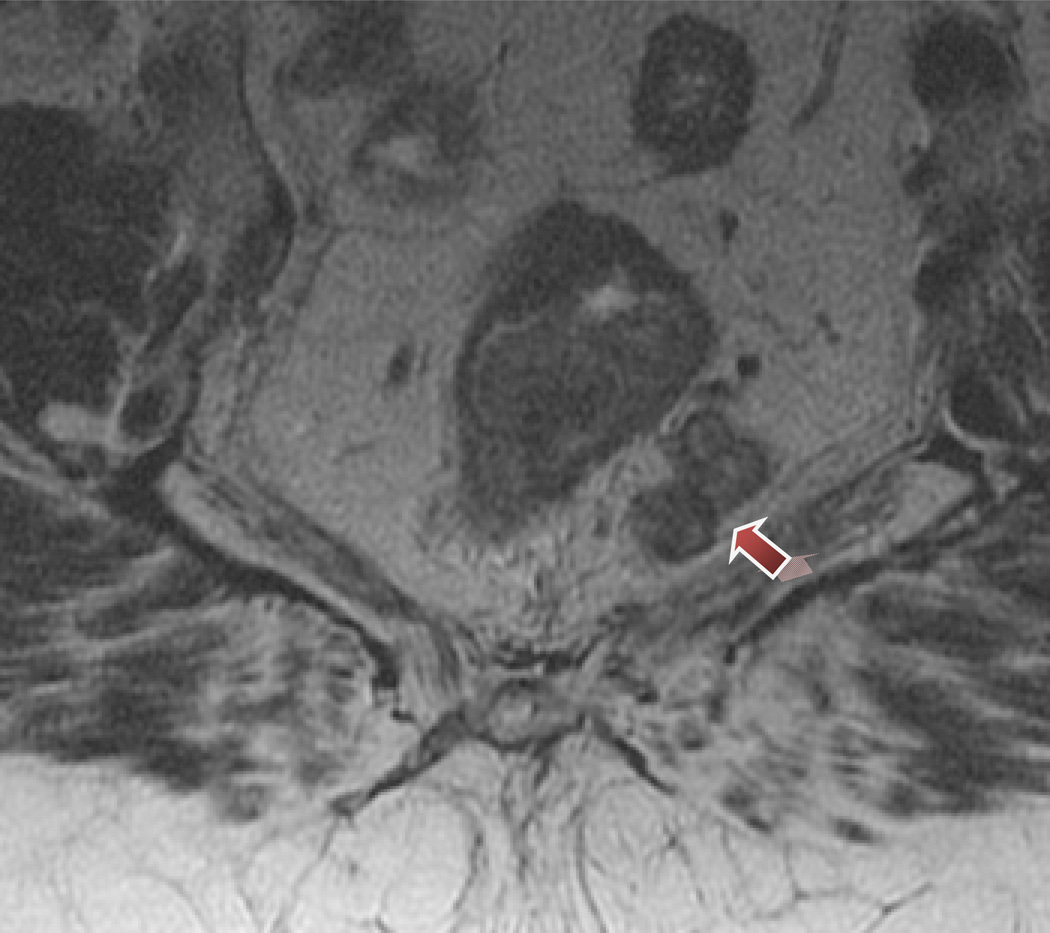
Figure 2.
Mixed signal intensity in metastatic mesorectal lymph nodes.
Histopathologic Results
Complete histopathologic mural tumor regression was identified in 15 (24.2%) patients, however 5 of these patients had 1-5 positive lymph, pathologic complete response rate of 16.1% (n=10). (Table 3). Among patients whose pathologic radial margin status was 1 mm or less, all patients had threatened radial margins on MRI. Among the 24 (38.1%) cases with MRI assessed threatened circumferential resection margins, the median pathologic radial margin distance was 5 mm [IQR 1–15 mm] and none of these patients achieved a pathologic complete response.
Table 3.
Final post-treatment histopathologic findings
| Variable | N (%) |
|---|---|
| pCR (ypT0N0) | 10 (16.1) |
| ypT1-2N0 | 15 (24.2) |
| ypT3-4 or N+ | 37 (59.7) |
| yp T stage | |
| T0 | 15 (24.2) |
| T1 | 7 (11.3) |
| T2 | 12 (19.4) |
| T3 | 23 (37.1) |
| T4 | 5 (8.1) |
| yp N stage | |
| N0 | 39 (62.9) |
| N1 | 15 (24.2) |
| N2 | 8 (12.9) |
| Lymphovascular invasion | 13 (21.0) |
| Perineural Invasion | 8 (12.9) |
| CRM (distance mm [IQR]) | 10 [5–15] |
| +ve (≤1 mm) | 7 (11.3) |
| -ve (>1 mm) | 55 (88.7) |
Univariate and Multivariate Analysis
The associations between the pre-treatment MRI and pathologic response are shown in tables 4 and 5. On univariate analysis, pre-treatment MRI findings of maximum tumor depth into the mesorectum, lymph node involvement and extramural vascular invasion but not grade were associated with poor treatment response. On multivariate analysis, MRI identified lymph node involvement and increasing tumor depth into the mesorectum remained associated with poor pathologic response to neoadjuvant therapy. The concordance index for the model was 0.86 demonstrating good discrimination. The presence of extramural vascular invasion on MRI was associated with both lymph node involvement and greater depth of mesorectal penetration and after adjustment for these other variables, was not independently associated with treatment response.
Table 4.
Univariate analysis of pre-operative factors associated with neoadjuvant treatment response.
| Tumor Characteristics | Good (ypT0-2N0) N=25 |
Poor (yp≥T3N0) N=37 |
OR | 95% CI | P |
|---|---|---|---|---|---|
| Mesorectal Tumor Depth | |||||
| <1 mm | 9 | 2 | 1 | ||
| 1–5 mm | 14 | 17 | 0.21 | 0.04–1.13 | 0.07 |
| >5 mm | 2 | 18 | 0.03 | 0.003–0.23 | 0.001 |
| Lymph Node Status | |||||
| Negative/Indeterminate | 18 | 6 | 1 | ||
| Positive | 7 | 31 | 0.075 | 0.02–0.26 | <0.001 |
| Vascular Invasion | |||||
| No | 24 | 22 | 1 | ||
| Yes | 1 | 15 | 0.06 | 0.007–0.50 | 0.009 |
| Grade | |||||
| Low | 21 | 27 | |||
| High | 4 | 10 | 0.51 | 0.14–1.87 | 0.31 |
Table 5.
Multivariate logistic regression analysis of preoperative factors associated with neoadjuvant treatment response (model concordance index=0.86).
| Tumor Characteristics | OR | 95% CI | P |
|---|---|---|---|
| Mesorectal Tumor Depth | |||
| < 1 mm | 1 | ||
| 1–5 mm | 0.29 | 0.04–2.04 | 0.22 |
| > 5 mm | 0.08 | 0.01–0.93 | 0.04 |
| Lymph Node Status | <0.001 | ||
| Negative/Indeterminate | 1 | ||
| Positive | 0.12 | 0.03–0.53 | 0.005 |
| Vascular Invasion | |||
| No | 1 | ||
| Yes | 0.70 | 0.05–9.76 | 0.79 |
| Grade | |||
| Low | 1 | ||
| High | 0.40 | 0.07–2.40 | 0.32 |
DISCUSSION
In this study we have demonstrated that high-resolution MRI staging for rectal cancer can be performed as an institutional policy within the U.S. and that the findings on pre-treatment MRI are associated with tumor response to neoadjuvant chemoradiation therapy. Greater depth of penetration into the mesorectum and MRI defined lymph node involvement were both independently associated with poor tumor response. Furthermore, in this group of patients with advanced disease, the identification of potential CRM involvement by MRI was associated with a final histopathologic diagnosis of radial margin distance ≤1 mm. The findings on pre-treatment MRI were therefore able to stratify patients as good or poor-risk for response to neoadjuvant chemoradiation therapy. This permits targeting of good or poor-risk patients for appropriate novel treatment strategies.
Although there has been prior interest in the use of MRI to identify high-risk rectal cancer patients for treatment stratification (e.g. immediate surgery versus preoperative radiotherapy), the ability of the preoperative MRI to risk-stratify neoadjuvant chemoradiation treatment response has not been previously considered. Primary surgical treatment without radiotherapy of “good prognosis” rectal cancers within the MERCURY study (clear CRM, no extramural venous invasion, tumor spread into mesorectum <5 mm) has been associated with a low rate of local failure13. However one limitation of this approach is that these findings are based on outcomes of MRI staging performed by specially trained radiologists followed by surgical resection performed by trained surgeons. Additional limitations may exist for generalizing these U.K. based study results to the U.S. population for whom MRI staging for rectal cancerhas not achieved general application. Furthermore, large-scale quality improvement programs for TME surgery would need to be employed, as have been conducted in the U.K., to ensure that patients selected for treatment with surgery alone undergo optimal oncologic resection. Thus the MERCURY study group data provides important preliminary results to stimulate further investigation of risk-stratified treatment strategies.
There has also been interest in identifying patients for consideration of intensified treatment strategies to improve resectibility of patients with poor-risk tumors 7, 8. Also poor treatment response appears to indicate of more aggressive tumor biology with poorer long-term outcomes than for patients with good response; and therefore poor-responders may benefit from intensified treatment strategies as well 14–16. The ability to risk-stratify patients for such treatments is dependent upon an ability to identify them prior to neoadjuvant treatment initiation. Traditionally this has been primarily based on the predicted status of the circumferential resection margin without information regarding the anticipated response to standard neoadjuvant chemoradiation therapy. Twenty-four patients(38.1%) in this study were noted to have threatened radial resection margins by pre-treatment MRI and 7 of these patients (29%) had yp margin status ≤ 1 mm, although none had a grossly positive margin. Among the remaining 17 patients (71%) in whom the margin was threatened on preoperative imaging, the median yp radial margin distance was 5 mm (IQR: 1–15). A total of 37 (59.7%) of the study patients were observed to have poor response to neoadjuvant therapy, however only 19 would have been identified by the identification of threatened radial margins. Furthermore, the composite criteria of lymph node status and depth of mesorectal penetration as defined in this study correctly identified 17 (94%). Thus the pre-treatment imaging can be used to identify high-risk (likely to have poor response)patients for novel treatment intensified protocols such as those incorporating induction chemotherapy or expanded radio sensitizing regimens. Given the potential additive toxicity of combination chemotherapy, the selection of poor-risk patients is important and limits the exposure to treatment-related toxicity for the good-risk groups.
For as long as MRI has been considered for rectal cancer staging, there has also been interest in the use of post-treatment MRI to identify complete responders to preoperative chemoradiotherapy. Patients with complete treatment response have been considered for organ-preserving non-surgical treatment strategies6. However treatment-associated changes to the primary tumor bed with fibrosis and collapse of the rectal wall can lead to difficulty in interpretation with a high degree of interobserver variability. In expert hands, a false-positive prediction for good response within the bowel wall (ypT0-2) will occur in 1 of 4 patients17. Although the addition of diffusion-weighted imaging can improve the evaluation and can decrease the interobserver variability particularly among radiologists with less experience in MRI for rectal cancer staging, accurate post-treatment lymph node assessment still remains a challenge and as of yet, there is no practical way to reliably identify complete responders18. Furthermore, the requirement for post-treatment imaging does not permit an opportunity for multidisciplinary treatment modification. Our results show that the pre-treatment high-resolution MRI yields useful information for neoadjuvant therapy response stratification. After adjustment for covariates, tumor depth >5 mm into the mesorectum was associated with 92% lower odds of good response and the identification of positive lymph nodes on MRI was associated with 88% lower odds of good response. Although the presence of extramural vascular invasion was an important univariate prognostic indicator on pre-treatment imaging, it was rarely identified in the absence of lymph node positivity or deep tumor penetration into the mesorectum and thus was not an independent predictor of response. In order to considernovel treatments that incorporateorgan-preserving wait-and-see type strategies for patients with pathologic complete response, it will be desirable to begin with a study cohort that has been enriched for potential responders based on pre-treatment MRI.
One current limitation to use of MRI for pre-treatment evaluation is the significant learning curve associated with image evaluation, and the uncertainty regarding nodal assessment, particularly in the era of long-course chemoradiation-associated nodal down-staging where there are limited opportunities for histopathologic confirmation of the pre-treatment radiographic assessment. At the University of Texas MD Anderson Cancer, we have incorporated routine high-resolution rectal MRI staging performed by a dedicated radiology team since September, 2009. The radiologist is present during image acquisition to ensure that the axial images are obtained perpendicular to the plane of the rectum at the level of the tumor. This may not be practical in all settings and alternative strategies for accurate imaging may need to be explored. A relatively unique feature of this cohort also was the disproportionately advanced tumor stage; a large number of patients had threatened circumferential resection margins and the majority of the patients were noted to have lymph node metastases on initial imaging. However, the rate of pathologic complete response was still 16%, consistent with previous reports from our institution and others14, 15.Also there may be inherent limitations of MRI such as to distinguish a T2 lesion from a very early T3 lesion with <1 mm mesorectal penetration. There were 11 patients in that category in this cohort, all of whom ultimately underwent neoadjuvant CXRT based on clinical nodal involvement. Recognizing this, we felt it was appropriate to focus on the final pathology stage as an overall indicator of response based on the clinical interpretation of the MRI that led to the recommendation for neoadjuvant therapy (e.g. stage mrII-III). Finally our analysis was designed not with the goal of definitively predicting pathologic complete response but rather with the aim to classify patients as highly or not likely to exhibit a good response to neoadjuvant therapy prior to treatment. Although it stands to reason that more advanced tumors were less likely to respond to neoadjuvant therapy, our results provide a semi-quantitative indicator of the extent to which the tumor burden can affect response.
CONCLUSION
Primary rectal cancer tumor characteristics on high-resolution MRI obtained prior to neoadjuvant chemoradiation are strongly associated with neoadjuvant treatment response. These factors should therefore be considered for stratification of patients for novel treatment strategies reliant on pathologic response to treatment or for the selection of poor-risk patients for intensified treatment regimens.
Footnotes
Disclosures:
1. Support to GJC by Career Development Awards from the American Society of Clinical Oncology Foundation Career Development Award and the National Cancer Institute K07-CA133187.
2. Podium presentation at the 2011 Annual Meeting of the American Society of Colon and Rectal Surgeons, Vancouver, British Columbia, Canada May14-18, 2011.
Author Contributions:
Conception and design: GJC, RDE
Data Acquisition: GJC, IP, RDE
Analysis and Interpretation: GJC, IP, HK, C-YH, RDE
Drafting of Manuscript: GJC, YNY, RDE
Critical Review: GJC, YNY, HK, MAR, JMS, RDE
REFERENCES
- 1.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, Calvo FA, Garcia-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W, Jr, Suarez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 3.Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Hoff PM, Eng C, Wolff RA, Janjan NA, Delclos ME, Krishnan S, Levy LB, Ellis LM, Crane CH. Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer. Am J Clin Oncol. 2006;29:219–224. doi: 10.1097/01.coc.0000214930.78200.4a. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Aguilar J, Hernandez de Anda E, Sirivongs P, Lee SH, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46:298–304. doi: 10.1007/s10350-004-6545-x. [DOI] [PubMed] [Google Scholar]
- 5.Borschitz T, Wachtlin D, Mohler M, Schmidberger H, Junginger T. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol. 2008;15:712–720. doi: 10.1245/s10434-007-9732-x. [DOI] [PubMed] [Google Scholar]
- 6.Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Jr, Silva e Sousa AH, Jr, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–717. doi: 10.1097/01.sla.0000141194.27992.32. discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, Tait D, Massey A, Tebbutt NC, Chau I. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241–248. doi: 10.1016/S1470-2045(09)70381-X. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, Vera R, Escudero P, Maurel J, Marcuello E, Mengual JL, Saigi E, Estevan R, Mira M, Polo S, Hernandez A, Gallen M, Arias F, Serra J, Alonso V. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859–865. doi: 10.1200/JCO.2009.25.8541. [DOI] [PubMed] [Google Scholar]
- 9.Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229–236. doi: 10.1002/bjs.5917. [DOI] [PubMed] [Google Scholar]
- 11.Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008;191:1827–1835. doi: 10.2214/AJR.08.1004. [DOI] [PubMed] [Google Scholar]
- 12.Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, Williams GT. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003;227:371–377. doi: 10.1148/radiol.2272011747. [DOI] [PubMed] [Google Scholar]
- 13.Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, Sebag-Montefiore DJ, Tekkis P, Brown G. Preoperative High-resolution Magnetic Resonance Imaging Can Identify Good Prognosis Stage I, II, and III Rectal Cancer Best Managed by Surgery Alone: A Prospective, Multicenter, European Study. Ann Surg. 2011;253:711–719. doi: 10.1097/SLA.0b013e31820b8d52. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CJ, Crane CH, Skibber JM, Rodriguez-Bigas MA, Chang GJ, Feig BW, Eng C, Krishnan S, Maru DM, Das P. Number of lymph nodes examined and prognosis among pathologically lymph node-negative patients after preoperative chemoradiation therapy for rectal adenocarcinoma. Cancer. 2011 doi: 10.1002/cncr.25973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silberfein EJ, Kattepogu KM, Hu CY, Skibber JM, Rodriguez-Bigas MA, Feig B, Das P, Krishnan S, Crane C, Kopetz S, Eng C, Chang GJ. Long-term survival and recurrence outcomes following surgery for distal rectal cancer. Ann Surg Oncol. 2010;17:2863–2869. doi: 10.1245/s10434-010-1119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang GJ, Rodriguez-Bigas MA, Eng C, Skibber JM. Lymph node status after neoadjuvant radiotherapy for rectal cancer is a biologic predictor of outcome. Cancer. 2009;115:5432–5440. doi: 10.1002/cncr.24622. [DOI] [PubMed] [Google Scholar]
- 17.Engelen SM, Beets-Tan RG, Lahaye MJ, Lammering G, Jansen RL, van Dam RM, Konsten J, Leijtens JW, van de Velde CJ, Beets GL. MRI after chemoradiotherapy of rectal cancer: a useful tool to select patients for local excision. Diseases of the colon and rectum. 2010;53:979–986. doi: 10.1007/DCR.0b013e3181dc64dc. [DOI] [PubMed] [Google Scholar]
- 18.Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M, Haustermans K, Valentini V, Beets GL, Beets-Tan RG. Diffusion-Weighted MRI for Selection of Complete Responders After Chemoradiation for Locally Advanced Rectal Cancer: A Multicenter Study. Annals of surgical oncology. 2011 doi: 10.1245/s10434-011-1607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]