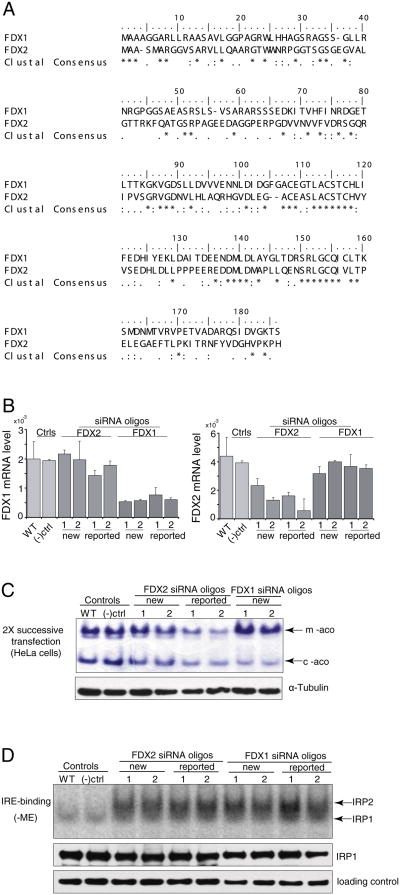
Fig. 1.
Silencing of either FDX1 or its homologue, FDX2, diminishes aconitase activities and activates iron-responsive element (IRE)-binding protein activities. (A) Protein sequence alignment of human FDX1 and FDX2. Identical residues are marked byasterisks, and similar residues are denoted by dots. (B) Comparison of mRNA change to evaluate specificity in FDX1 or FDX2 knock-downs in HeLa cells. (C) In-gel aconitase assays revealed activity of mitochondrial and cytosolic aconitases in HeLa cells treated with FDX1 and FDX2 siRNA (WT, wild-type; (–)ctrl, negative control; oligo, siRNA of either FDX1 or FDX2) after two successive transfections. (D) Gel retardation assays of IRPs. Transfected cells were harvested six days after two successive transfections and analyzed for total binding activity of IRP1 and IRP2 to 32P-labeled IRE of human ferritin mRNA. Lysates of WT, FDX1 and FDX2 knock-down HeLa cells (10 mg protein/lane) were incubated with 32P-IRE and resolved on a 10% non-denaturing gel. Lanes were: WT, wild type; (–) ctrl, negative control; oligo, siRNAs of FDX1 or FDX2 as labeled. IRP1 is the bottom band and IRP2 is the upper band of the IRP-IRE complexes. Western blots for IRP1 and α-tubulin (loading control) demonstrate equal loading of the gels.