Abstract
Arterial and venous thromboembolic diseases are a clinical and economic burden worldwide. In addition to traditional agents such as vitamin K antagonists and heparins, newer oral agents – such as the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban, and the direct thrombin inhibitor dabigatran – have been shown to be effective across several indications. Rivaroxaban has been shown to have predictable pharmacokinetic and pharmacodynamic properties, including a rapid onset of action. In addition, there is no requirement for routine coagulation monitoring; and no dose adjustment is necessary for age alone, sex, or body weight. Rivaroxaban has successfully met primary efficacy and safety endpoints in large, randomized phase III trials across several indications, including: prevention of venous thromboembolism in orthopedic patients undergoing elective hip or knee replacement surgery; treatment of deep vein thrombosis and secondary prevention of deep vein thrombosis and pulmonary embolism; stroke prevention in patients with atrial fibrillation; and secondary prevention of acute coronary syndrome. Rivaroxaban and the other newer oral anticoagulants are likely to improve outcomes in the prevention and treatment of thromboembolic events, and will offer patients and physicians alternative treatment options.
Keywords: acute coronary syndrome, atrial fibrillation, orthopedics, pulmonary embolism, rivaroxaban, stroke, venous thromboembolism, venous thrombosis
Introduction
Arterial and venous thromboembolic disease is common and imposes a large clinical and economic burden on healthcare systems, with a substantial effect on patients’ quality of life. Venous thromboembolism (VTE) comprises deep vein thrombosis (DVT) and pulmonary embolism (PE). The annual rate of venous thromboembolic events or deaths in six European Union (EU) countries was estimated at 1 million [Cohen et al. 2007]; in the USA, the annual rate of nonfatal symptomatic venous thromboembolic events was estimated at more than 600,000 [Heit et al. 2005].
For arterial thromboembolic disease, both atrial fibrillation (AF) and acute coronary syndrome (ACS) contribute to substantial levels of morbidity and mortality across the world. AF is the most common cardiac arrhythmia. It occurs in 1–2% of the general population and is responsible for 20% of strokes [Camm et al. 2010; Lemmens et al. 2011]. Cardiovascular diseases, including ACS, are currently the leading cause of death in industrialized countries and represent a sizable demand on healthcare resources in the EU and USA [Murray and Lopez, 1997].
In view of the prevalence of thromboembolic disorders, which is in part due to an increasing number of older people, more effective prevention and treatment will offer substantial benefits to healthcare systems, physicians, and patients. In the past 5 years, the range of anticoagulation options available to physicians has increased substantially.
In addition to traditional agents, such as heparins, low molecular weight heparins (LMWHs), fondaparinux, and vitamin K antagonists (VKAs), a number of new oral anticoagulants have now received approval for a variety of indications. These newer agents target either thrombin (dabigatran) or factor Xa (rivaroxaban, apixaban, and edoxaban). Thrombin has a central role in blood coagulation and thrombus formation through the conversion of fibrinogen to fibrin. The direct thrombin inhibitor dabigatran occupies the catalytic binding site of thrombin or the fibrinogen-binding site, directly neutralizing thrombin. Factor Xa has a central role in the coagulation cascade; it is involved in the generation of thrombin and is an attractive drug target. Factor Xa inhibitors bind directly to the active site of factor Xa to inhibit thrombin generation. These agents have initiated a new era for anticoagulation therapy, due in part to the convenience of oral administration and to their predictable pharmacokinetic and pharmacodynamic properties compared with traditional agents [Weitz et al. 2008]. VKAs, such as warfarin, have a narrow therapeutic range, require regular monitoring, and present challenges in achieving optimal anticoagulation [Ansell et al. 2008]. LMWHs and fondaparinux are widely used for VTE prevention and exhibit predictable pharmacokinetic and pharmacodynamic properties; however, parenteral administration of these agents can be inconvenient for long-term outpatient use [Hirsh et al. 2008; Nutescu, 2003].
Approval of the newer oral anticoagulants has been based on the results of several successful phase III studies in the prevention and treatment of thromboembolic disorders [Connolly et al. 2009; Eriksson et al. 2007a, 2007b, 2008; Kakkar et al. 2008; Lassen et al. 2008, 2010a, 2010b; Patel et al. 2011; The EINSTEIN Investigators, 2010; Turpie et al. 2009]. The focus of this review is rivaroxaban: the oral, direct factor Xa inhibitor that has received approval for use across a broad range of indications. Rivaroxaban has been the subject of a large clinical development program involving more than 75,000 patients. To date, rivaroxaban has received approval in many countries for the prevention of VTE in adult patients undergoing elective hip or knee replacement surgery. Approval has also been granted for the treatment of DVT and secondary prevention of DVT and PE following an acute DVT in adults (EU and Canada), and for the prevention of stroke and systemic embolism in adult patients with AF (EU, Canada, Japan, and the USA).
Target and pharmacological properties of rivaroxaban
Factor Xa is an attractive drug target in the coagulation cascade because it is involved in both the initiation and propagation of the coagulation process and the conversion of prothrombin to thrombin. Rivaroxaban binds tightly to the active site of factor Xa [inhibition constant (Ki) = 0.4 nM], leading to an inactivation of free and fibrin-bound factor Xa, as well as factor Xa, within the prothrombinase complex [Perzborn et al. 2005; Samama, 2011].
Rivaroxaban has exhibited predictable pharmacokinetics and pharmacodynamics in healthy subjects, in single or multiple doses (5–80 mg/day), with no clinically relevant changes in bleeding time or other safety parameters [Kubitza et al. 2005b, 2005c]. Rivaroxaban exhibits dose-proportional pharmacokinetics, with a high oral bioavailability of 80–100% (after administration of a 10 mg dose), and a half life of 5–13 h (5–9 h in young individuals; 11–13 h in older people) [Bayer Pharma AG, 2011; Kubitza et al. 2005b, 2005c, 2008]. Maximum inhibition of factor Xa activity occurred approximately 3 h after oral administration, which correlates with time to peak plasma concentration (2–4 h), with no relevant accumulation observed at any approved dose [Kubitza et al. 2005b, 2005c]. Phase I studies in healthy subjects showed that single doses of rivaroxaban had pharmacodynamic effects that persisted for 24 h [Harder et al. 2004; Kubitza et al. 2005a, 2005b]. In addition, rivaroxaban significantly inhibited peak and total amounts of thrombin generated and prolonged time to thrombin generation 24 h after dosing in healthy subjects [Harder et al. 2004]. Elimination is by multiple routes; one-third of the drug is excreted unchanged as active drug in the urine; two-thirds of the drug is metabolized in the liver, half of which is then eliminated via the kidneys and half via the hepatobiliary route [Bayer Pharma AG, 2011].
The recommended doses of rivaroxaban for each indication are as follows: prevention of VTE in orthopedic patients undergoing elective hip or knee replacement surgery, 10 mg once daily; treatment of DVT and secondary prevention of DVT and PE, 15 mg twice daily (for the first 3 weeks) followed by 20 mg once daily (for continued treatment); prevention of stroke and systemic embolism in patients with AF, 20 mg once daily [Bayer Pharma AG, 2011]. No dose adjustment is necessary in patients with mild renal impairment [creatinine clearance (CrCl) 50–80 ml/min). In patients with moderate (CrCl 30–49 ml/min) and severe (CrCl 15–29 ml/min) renal impairment, a reduced dose of 15 mg is recommended for the treatment of DVT and secondary prevention of DVT and PE (15 mg twice daily for the first 3 weeks; 15 mg once daily for continued treatment), and the prevention of stroke and systemic embolism in patients with AF (15 mg once daily). There are limited clinical data regarding the use of rivaroxaban in patients with severe renal impairment. Rivaroxaban is not recommended in patients who have CrCl <15 ml/min [Bayer Pharma AG, 2011]. In addition, rivaroxaban does not require dose adjustment for age, body weight, or sex, and has a low risk of drug–drug interactions with commonly used concomitant medications including acetylsalicylic acid (ASA), atorvastatin, and naproxen [Bayer Pharma AG, 2011]. Coadministration of rivaroxaban with clopidogrel has been associated with an increase in bleeding time in some healthy subjects; however, an increase in bleeding time in healthy subjects has been shown with other combination therapies, such as ASA and clopidogrel [Bayer Pharma AG, 2011; Payne et al. 2002].
Clinical studies of rivaroxaban
Phase III studies of rivaroxaban have been completed in VTE prevention after total hip replacement (THR) and total knee replacement (TKR) [Regulation of Coagulation in Orthopaedic Surgery to Prevent Deep Vein Thrombosis and Pulmonary Embolism (RECORD) 1–4] [Eriksson et al. 2008; Kakkar et al. 2008; Lassen et al. 2008; Turpie et al. 2009], VTE prevention in acutely ill medical patients [Multicenter, Randomized, Parallel Group Efficacy and Safety Study for the Prevention of Venous Thromboembolism in Hospitalized Medically Ill Patients Comparing Rivaroxaban with Enoxaparin (MAGELLAN)] [Cohen et al. 2011a], treatment of acute symptomatic DVT and PE (EINSTEIN DVT, EINSTEIN PE) [The EINSTEIN Investigators, 2010; The EINSTEIN–PE Investigators, 2012], prevention of recurrent VTE (EINSTEIN Extension) [The EINSTEIN Investigators, 2010], stroke prevention in patients with nonvalvular AF [Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF)] [Patel et al. 2011], and secondary prevention of ACS [Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Aspirin with/without Thienopyridine Therapy in Subjects with Acute Coronary Syndrome – Thrombolysis in Myocardial Infarction 51 (ATLAS ACS 2 TIMI 51)] [Mega et al. 2012].
Venous thromboembolism prevention in patients undergoing hip or knee replacement surgery
Patients have a significant risk of VTE after orthopedic and other forms of surgery. Patients who undergo orthopedic surgery without prophylaxis have been shown to develop DVT in 40–60% of cases [Geerts et al. 2008].
Anticoagulation therapy is recommended by the American College of Chest Physicians (ACCP) for patients undergoing THR/TKR for 10–35 days [Geerts et al. 2008]. There is evidence that extended anticoagulant prophylaxis for 1 month reduces the rate of symptomatic venous thromboembolic events and is more effective than prophylaxis administered during hospitalization [Bergqvist et al. 1996]. There is also evidence suggesting that thromboprophylaxis is underused [Huo and Spyropoulos, 2011].
Data from a phase II, dose-escalation study investigating the efficacy and safety of rivaroxaban in patients undergoing THR showed that 10 mg once daily was the most favorable dose regimen for the phase III studies in orthopedic surgery patients [Eriksson et al. 2006]. The RECORD phase III program assessed the safety and efficacy of rivaroxaban compared with enoxaparin for the prevention of VTE in four multicenter, randomized, double-blind trials in patients undergoing elective THR (RECORD1 and 2) or TKR (RECORD3 and 4) [Eriksson et al. 2008; Kakkar et al. 2008; Lassen et al. 2008; Turpie et al. 2009].
RECORD1 compared 31–39 days of rivaroxaban prophylaxis with 31–39 days of enoxaparin prophylaxis after THR surgery, whereas RECORD2 compared 31–39 days of rivaroxaban prophylaxis with 10–14 days of enoxaparin prophylaxis followed by placebo, after THR surgery. RECORD3 and RECORD4 compared 10–14 days of rivaroxaban prophylaxis with 10–14 days of enoxaparin prophylaxis (40 mg once daily and 30 mg twice daily, respectively) in patients undergoing TKR surgery. The RECORD study designs, and efficacy and safety outcomes, are shown in Table 1.
Table 1.
Study design, efficacy endpoints, and safety outcomes in the phase III RECORD studies of rivaroxaban for the prevention of VTE after THR or TKR surgery.
| Study | Design | Population | N * | Regimen | Duration | Endpoints/outcomes† |
|---|---|---|---|---|---|---|
| RECORD1 | Double blind, double dummy | THR | 4541 | Rivaroxaban 10 mg once daily versus enoxaparin 40 mg once daily | Rivaroxaban 31–39 days Enoxaparin 31–39 days |
Efficacy endpoints
•Primary: total VTE [composite of any DVT, nonfatal PE, and all-cause mortality up to day 42 (RECORD1 and 2) or day 17 (RECORD3 and 4)] •Secondary: major VTE (composite of proximal DVT, nonfatal PE, and VTE-related death) •Other: incidence of any DVT, the incidence of symptomatic VTE during the active therapy and follow-up periods, and death during the follow-up period |
|
| ||||||
| RECORD2 | Double blind, double dummy | THR | 2509 | Rivaroxaban 10 mg once daily versus enoxaparin 40 mg once daily | Rivaroxaban 31–39 days Enoxaparin 10–14 daysfollowed byplacebo |
|
|
| ||||||
| RECORD3 | Double blind, double dummy | TKR | 2531 | Rivaroxaban 10 mg once daily versus enoxaparin 40 mg once daily | Rivaroxaban 10–14 days Enoxaparin 10–14 days |
|
|
| ||||||
| RECORD4 | Double blind, double dummy | TKR | 3148 | Rivaroxaban 10 mg once daily versus enoxaparin 30 mg twice daily | Rivaroxaban 10–14 days Enoxaparin 10–14 days |
Safety outcomes
•Bleeding occurring after the first blinded dose of study drug and no later than 2 days after the last dose •Primary: major bleeding (bleeding that was fatal, into a critical organ, or required re-operation; extra-surgical-site bleeding associated with a drop in hemoglobin ≥2 g/dl, or requiring transfusion of≥2 units of blood) •Other: any on-treatment bleeding, nonmajor bleeding, nonmajor clinically relevant bleeding, hemorrhagic wound complications, cardiovascular adverse events, liver enzyme levels |
Randomized.
As assessed by central independent adjudication committees.
DVT, deep vein thrombosis; PE, pulmonary embolism; RECORD, Regulation of Coagulation in Orthopaedic Surgery to Prevent Deep Vein Thrombosis and Pulmonary Embolism; THR, total hip replacement; TKR, total knee replacement; VTE, venous thromboembolism.
Across the RECORD studies, the primary efficacy endpoint was total VTE: the composite of any DVT, nonfatal PE, and all-cause mortality. The incidence of total VTE was significantly reduced by treatment with rivaroxaban compared with enoxaparin in the RECORD1–4 studies (Figure 1(a)). The incidence of major VTE (composite of proximal DVT, nonfatal PE, and VTE-related death) was also significantly reduced compared with enoxaparin in RECORD1, 2, and 3 (RECORD1: 0.2% versus 2.0%, p < 0.001; RECORD2: 0.6% versus 5.1%, p < 0.0001; RECORD3: 1.0% versus 2.6%, p = 0.01). The occurrence of symptomatic VTE was lower across all studies in the rivaroxaban group compared with the enoxaparin group (RECORD1: 0.3% versus 0.5%, p = 0.22; RECORD2: 0.2% versus 1.2%, p = 0.0040; RECORD3: 0.7% versus 2.0%, p = 0.005; RECORD4: 0.7% versus 1.2%, p = 0.1868) [Eriksson et al. 2008; Kakkar et al. 2008; Lassen et al. 2008; Turpie et al. 2009].
Figure 1.
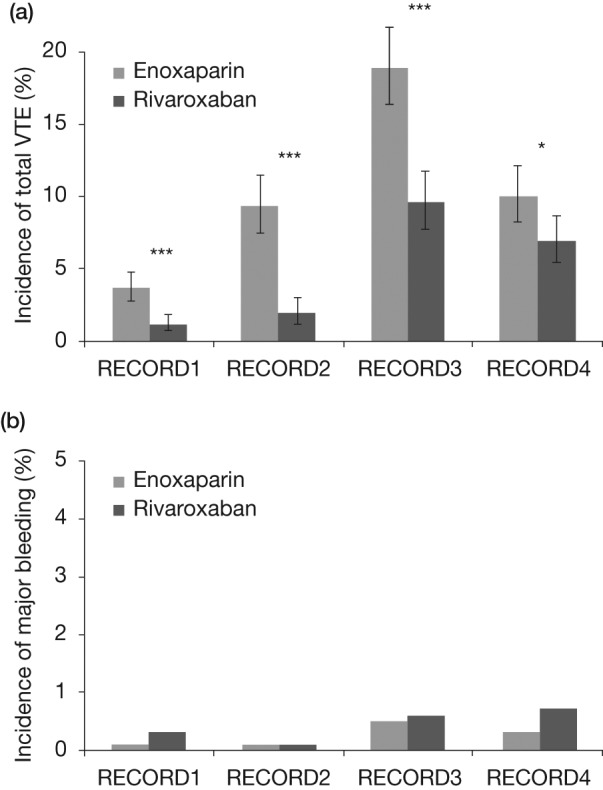
(a) Primary efficacy outcome (total VTE) in orthopedic patients in the RECORD program. Rates of total VTE were significantly lower with rivaroxaban than with enoxaparin in RECORD1–4. RECORD1: 1.1% versus 3.7%, p < 0.001; RECORD2: 2.0% versus 9.3%, p < 0.0001; RECORD3: 9.6% versus 18.9%, p < 0.001; RECORD4: 6.9% versus 10.1%, p = 0.0118. *p < 0.05; **p < 0.01; ***p < 0.001. (b) Incidence of major bleeding events in orthopedic patients in the RECORD program. Major bleeding was low in the rivaroxaban and enoxaparin arms and rates were not significantly different between groups. RECORD1: 0.3% versus 0.1%, p = 0.18; RECORD2: <0.1% for both groups; RECORD3: 0.6% versus 0.5%, p = 0.77; RECORD4: 0.7% versus 0.3%, p = 0.1096. RECORD, Regulation of Coagulation in Orthopaedic Surgery to Prevent Deep Vein Thrombosis and Pulmonary Embolism; VTE, venous thromboembolism.
Rates of major bleeding (defined as bleeding that was fatal, into a critical organ, or required reoperation; or extra-surgical-site bleeding associated with a drop in hemoglobin ≥2 g/dl or requiring transfusion of ≥2 units of blood) were low in the rivaroxaban and enoxaparin arms and were not significantly different (Figure 1(b)). The composite of major and nonmajor clinically relevant bleeding across the RECORD program was higher with rivaroxaban compared with enoxaparin (RECORD1: 3.2% versus 2.5%; RECORD2: 3.4% versus 2.8%; RECORD3: 3.3% versus 2.7%, p = 0.44; RECORD4: 3.0% versus 2.3%, p = 0.1790). There were no cases of fatal bleeding in RECORD2 or RECORD3; one case was reported in the rivaroxaban group in RECORD1 and RECORD4 overall [Eriksson et al. 2008; Kakkar et al. 2008; Lassen et al. 2008; Turpie et al. 2009].
Adverse event rates were similar between the rivaroxaban and enoxaparin groups in all four RECORD studies. There was no evidence of liver safety issues, based on plasma alanine aminotransferase [ALT; >3× upper limit of normal (ULN)] and serum bilirubin levels (>2× ULN) measurements. Cardiovascular event rates were also low across all studies and were shown to be similar between the rivaroxaban and enoxaparin arms (RECORD1: 0.2% versus 0.4%; RECORD2: 0.7% versus 0.3%; RECORD3: 0.3% versus 0.2%; RECORD4: 0.5% versus 0.7%). Postoperative wound infection rates were also similar between the rivaroxaban and enoxaparin arms (RECORD1: 0.4% in both groups; RECORD2: 0.7% versus 0.5%; RECORD3: 0.6% versus 0.9%; RECORD4: 0.3% versus 0.2%) [Eriksson et al. 2008; Kakkar et al. 2008; Lassen et al. 2008; Turpie et al. 2009]. Overall, results from the RECORD program showed the superior efficacy and similar safety of rivaroxaban compared with enoxaparin in the prevention of VTE after elective THR or TKR surgery.
A pooled analysis of the RECORD1–3 studies was performed to investigate the efficacy of rivaroxaban (10 mg once daily) compared with enoxaparin (40 mg once daily) in reducing symptomatic VTE and all-cause mortality [Eriksson et al. 2009]. The primary endpoint of the analysis was the composite of VTE (symptomatic DVT and nonfatal PE) and all-cause mortality up to 2 weeks. Rivaroxaban demonstrated a significant reduction in the incidence of symptomatic VTE compared with enoxaparin [0.4% versus 0.8%, odds ratio (OR) 0.44, p = 0.005]. For the secondary efficacy endpoint [symptomatic VTE and all-cause mortality up to 2 weeks (RECORD3) or up to 5 weeks (RECORD1 and 2)], further significant reductions were observed (0.5% versus 1.3%, OR = 0.38; p < 0.001). There was also a reduction in the composite of all-cause mortality and PE in patients receiving rivaroxaban compared with patients receiving enoxaparin (0.3% versus 0.7%, OR = 0.52, p = 0.042). Rates of major bleeding were similar up to 2 weeks (0.2% for both groups) and for the planned treatment period (rivaroxaban 0.3%; enoxaparin 0.2%) [Eriksson et al. 2009].
A further pooled analysis of the RECORD1–4 (12,729 patients) studies investigated the effects of rivaroxaban on symptomatic VTE and all-cause mortality compared with enoxaparin (30 mg twice daily or 40 mg once daily), in addition to safety outcomes [Turpie et al. 2011]. For the analysis, data were used from the day 12±2 active treatment pool to account for the different study designs (Table 1). The results confirmed the superior efficacy of rivaroxaban in the reduction of symptomatic VTE and all-cause mortality in THR and TKR surgery patients compared with enoxaparin (0.5% versus 1.0%, p = 0.001). Rates of major bleeding were similar between rivaroxaban and enoxaparin in this large cohort of patients (0.3% versus 0.2%, p = 0.23), in addition to rates of major and nonmajor clinically relevant bleeding (2.8% versus 2.5%, p = 0.19) and any bleeding (6.6% versus 6.2%, p = 0.38).
A range of bleeding definitions has been used in clinical trials. The RECORD1–4 pooled analysis compared rates of bleeding between rivaroxaban and enoxaparin regimens using three different definitions of bleeding. These definitions were from the International Society on Thrombosis and Haemostasis (ISTH; inclusion of clinically overt surgical-site bleeding events), the European Medicines Agency (EMA; inclusion of clinically overt surgical-site bleeding events and bleeding warranting treatment cessation), and those used in the RECORD program [Turpie et al. 2011]. In all cases, there was no significant difference in rates of major bleeding between the rivaroxaban and enoxaparin regimens when these different definitions were used (ISTH: rivaroxaban 1.7%, enoxaparin 1.4%, OR = 1.29; EMA: rivaroxaban 2.0%, enoxaparin 1.7%, OR = 1.20).
Venous thromboembolism prevention in acutely ill medical patients
Medical conditions, such as acute heart failure, acute infection and acute respiratory failure increase the risk of VTE and this risk can be exacerbated by prolonged periods of immobilization and other factors, including advanced age and prior VTE [Leizorovicz and Mismetti, 2004]. Anticoagulation is recommended for acutely ill patients at risk of VTE, but the optimal duration of therapy is not known. The MAGELLAN study compared extended duration rivaroxaban 10 mg once daily for 35±4 days with standard duration enoxaparin 40 mg once daily for 10±4 days followed by placebo [Cohen et al. 2011a]. Extended prophylaxis significantly decreased the risk of VTE compared with standard prophylaxis but was associated with significantly higher rates of bleeding [Cohen et al. 2011b]. Other contemporary studies of extended prophylaxis in acutely ill medical patents have also shown increased risk of bleeding [Goldhaber et al. 2011; Hull et al. 2010], suggesting that the potential benefits of prolonging prophylaxis in these patients are outweighed by the risks.
Venous thromboembolism treatment and secondary prevention
VTE affects more than 1 million individuals per year in the EU [Cohen et al. 2007]. In the USA, this figure exceeds 600,000 people [Heit et al. 2005]. Thromboembolic diseases are increasing in prevalence and, for VTE, the number of adults with the disease is projected to double from 0.95 million in 2006 to 1.82 million in 2050 [Deitelzweig et al. 2011]. After an initial venous thromboembolic episode, patients are at increased risk of recurrence, and although this risk diminishes with time it never disappears [Prandoni et al. 2007; Rodger et al. 2008]. A number of risk factors have been identified for VTE [Geerts et al. 2001], and anticoagulation therapy is used in initial treatment and longer-term secondary prevention.
Standard treatment regimens include a parenteral anticoagulant (such as LMWH, unfractionated heparin, or fondaparinux) for approximately 5–7 days, combined with a VKA until the international normalized ratio reaches at least 2.0 for at least 24 h [Kearon et al. 2008]. Current VTE treatment options involving a dual-drug approach of parenteral anticoagulant plus a VKA are effective but there are disadvantages, such as the risk of heparin-induced thrombocytopenia with unfractionated heparin [Hirsh et al. 2008] and the need for regular coagulation monitoring and dose adjustment with VKAs [Ansell et al. 2008].
Phase II studies with rivaroxaban confirmed that both 15 mg twice daily and 20 mg once daily doses were optimal for the treatment of patients with acute symptomatic DVT [Agnelli et al. 2007; Büller et al. 2008], which led to their use in the phase III EINSTEIN program. The EINSTEIN program comprised three, randomized, event-driven studies in which rivaroxaban was compared with either the standard of care (enoxaparin/VKA; EINSTEIN DVT and EINSTEIN PE) [The EINSTEIN Investigators, 2010; The EINSTEIN–PE Investigators, 2012] or placebo (EINSTEIN Extension) [The EINSTEIN Investigators, 2010]. In the EINSTEIN DVT and EINSTEIN PE studies, a single-drug approach was used, and parenteral therapy was not given as initial anticoagulation. However, patients were allowed to receive up to 48 h of treatment with LMWH during the diagnostic workup. EINSTEIN DVT was a randomized, open-label, event-driven noninferiority study in patients with acute, symptomatic DVT; EINSTEIN Extension was a randomized, double-blind, event-driven superiority study in patients with confirmed symptomatic DVT or PE who had received 6–12 months’ treatment with acenocoumarol, warfarin, or rivaroxaban. In the EINSTEIN PE study, rivaroxaban was investigated in patients with confirmed acute symptomatic PE with or without symptomatic DVT. The EINSTEIN study designs, including a description of efficacy and safety outcomes, are shown in Table 2.
Table 2.
Study design, efficacy endpoints, and safety outcomes in the phase III EINSTEIN studies of rivaroxaban for the prevention of VTE.
| Study | Design | Population | N | Regimen | Duration | Endpoints |
|---|---|---|---|---|---|---|
| EINSTEIN DVT | Open label | Patients with acute, symptomatic objectively confirmed proximal DVT without symptomatic PE | 3449 | Rivaroxaban 15 mg twice daily for 3 weeks, followed by 20 mg once daily | Rivaroxaban for 3, 6, or 12 months |
Efficacy endpoints
•Primary: symptomatic, recurrent VTE (the composite of DVT or fatal or nonfatal PE) |
| Enoxaparin 1 mg/kg body weight twice daily plus a VKA (warfarin or acenocoumarol), followed by dose-adjusted VKA only (target INR of 2.0–3.0) | Enoxaparin plus VKA continued until INR ≥2.0 for 2 consecutive days, and ≥5 days of enoxaparin therapy had been administered; followed by dose-adjusted VKA (target INR of 2.0–3.0) for the remainder of the treatment period (a total of 3, 6, or 12 months) |
Safety outcomes
•Primary: clinically relevant bleeding (the composite of major* or nonmajor† clinically relevant bleeding) |
||||
|
| ||||||
| EINSTEIN Extension | Double blind | Patients with objectively confirmed symptomatic DVT/PE, treated for 6–12 months with acenocoumarol, warfarin, or rivaroxaban, if there was equipoise with respect to the need for continued anticoagulation | 1197 | Rivaroxaban 20 mg once daily Placebo |
Rivaroxaban for 6 or 12 months Placebo for 6 or 12 months |
Efficacy endpoints
•Primary: symptomatic, recurrent VTE (the composite of DVT or nonfatal or fatal PE) Safety outcomes •Primary: major bleeding |
|
| ||||||
| EINSTEIN PE | Open label | Patients with acute, symptomatic, objectively confirmed PE with or without symptomatic DVT | 4832 | Rivaroxaban 15 mg twice daily for 3 weeks, followed by 20 mg once daily | Rivaroxaban for 3, 6, or 12 months |
Efficacy endpoints
•Primary: symptomatic, recurrent VTE (the composite of DVT or fatal or nonfatal PE) |
| Enoxaparin 1 mg/kg body weight twice daily plus a VKA (warfarin or acenocoumarol), followed by dose-adjusted VKA only (target INR of 2.0–3.0) | Enoxaparin plus VKA continued until INR ≥2.0 for 2 consecutive days, and ≥5 days of enoxaparin therapy had been administered; followed by dose-adjusted VKA (target INR of 2.0–3.0) for the remainder of the treatment period (a total of 3, 6, or 12 months) |
Safety outcomes
•Primary: clinically relevant bleeding (the composite of major* or nonmajor† clinically relevant bleeding) |
||||
Clinically overt and associated with a fall in hemoglobin level of ≥2 g/dl, if it led to transfusion of ≥2 units of red cells, or if it was retroperitoneal, intracranial, occurred in a critical site, or contributed to death.
Nonmajor clinically relevant bleeding was defined as overt bleeding not meeting the criteria for major bleeding but associated with medical intervention, unscheduled contact with a physician, interruption or discontinuation of study treatment, or associated with any other discomfort such as pain or impairment of activities of daily life.
DVT, deep vein thrombosis; INR, international normalized ratio; PE, pulmonary embolism; VKA, vitamin K antagonist; VTE, venous thromboembolism.
In the EINSTEIN DVT study, the primary efficacy endpoint of symptomatic, recurrent VTE occurred in 2.1% of patients receiving rivaroxaban and 3.0% of patients receiving standard therapy (p < 0.001 for noninferiority) [The EINSTEIN Investigators, 2010]. Rates of major bleeding and nonmajor clinically relevant bleeding were similar between the groups (8.1% for both groups, p = 0.77); rates of major bleeding alone were 0.8% in patients receiving rivaroxaban and 1.2% in patients receiving enoxaparin/VKA (p = 0.21). A net clinical benefit (a composite of VTE plus major bleeding) was observed in 2.9% of patients receiving rivaroxaban compared with 4.2% of patients who received enoxaparin/VKA (p = 0.03). Liver safety parameters were similar in the rivaroxaban and enoxaparin/VKA groups, where elevated levels of plasma ALT (>3× ULN) and serum bilirubin (>2× ULN) were seen in 0.1% versus 0.2% of patients, respectively.
In the EINSTEIN Extension study, the primary efficacy endpoint of symptomatic, recurrent VTE occurred in 1.3% of patients receiving rivaroxaban and 7.1% of patients receiving placebo (p < 0.001 for superiority), which translated into a relative risk reduction of 82%. Rates of major bleeding were low in both groups (rivaroxaban 0.7%; placebo 0.0%; p = 0.11), whereas rates of nonmajor clinically relevant bleeding were 5.4% in patients receiving rivaroxaban compared with 1.2% in those receiving placebo. EINSTEIN Extension showed that the outcome of a net clinical benefit (a composite of symptomatic, recurrent VTE plus major bleeding) occurred in 2.0% of patients receiving rivaroxaban and in 7.1% of those receiving placebo (p < 0.001). Liver safety parameters were not affected in either treatment group: no clinically significant elevations of plasma ALT (>3× ULN) or serum bilirubin (>2× ULN) were observed.
In the EINSTEIN PE study, the primary efficacy outcome of symptomatic, recurrent VTE (composite of fatal or nonfatal PE or DVT) occurred in 2.1% of patients receiving rivaroxaban and in 1.8% of those receiving standard therapy (p = 0.003 for a one-sided noninferiority margin of 2.0; p = 0.57 for superiority) [The EINSTEIN–PE Investigators, 2012]. The principal safety outcome was clinically relevant bleeding, defined as a composite of major or nonmajor clinically relevant bleeding. Rates of major or nonmajor clinically relevant bleeding were comparable in patients receiving rivaroxaban and patients receiving enoxaparin/VKA (10.3% versus 11.4%, respectively; p = 0.23); however, the rate of major bleeding was significantly lower in patients receiving rivaroxaban compared with patients receiving enoxaparin/VKA (1.1% versus 2.2%, respectively; p = 0.003). The outcome of a net clinical benefit (a composite of symptomatic recurrent VTE plus major bleeding) occurred in 3.4% of patients receiving rivaroxaban and in 4.0% of those receiving enoxaparin/VKA (p = 0.28). In EINSTEIN PE, the number of acute coronary events was similarly low in both treatment arms, as were changes in liver safety parameters.
Collectively, the EINSTEIN studies showed that rivaroxaban was effective when used as a single agent in the treatment of acute DVT, acute PE, and the prevention of recurrent VTE. Rivaroxaban was associated with an improved net clinical benefit for both acute and longer-term treatment and had an acceptable safety profile, with no significant increase in major bleeding or adverse events.
Stroke prevention in patients with atrial fibrillation
AF is the most common arrhythmia seen in clinical practice and is associated with a fivefold increase in the risk of stroke [Wolf et al. 1991]. The prevalence of AF increases with age and has been shown to occur in 8.8% of patients aged 80–89 years compared with 0.5% in patients aged 50–59 years [Lloyd-Jones et al. 2010]. More than 6 million Europeans have AF and it is predicted that the rate will double in the next 50 years, with an associated increase in the number of strokes [Camm et al. 2010]. In the EU, the number of annual deaths related to all strokes is estimated at 1.24 million, and the financial burden is substantial [Rayner et al. 2009; Roger et al. 2011]. In the USA, the annual cost of stroke was estimated at $40.9 billion in 2007 [Roger et al. 2011] and, because of the burden associated with stroke, more effective therapy is required. Despite the fact that both clinical trials and extensive worldwide use show the effective role of VKAs in the treatment of patients with AF, the limitations associated with their use have led to the search for therapeutic alternatives.
ROCKET AF was a multicenter, randomized, double-blind, double-dummy, event-driven trial that compared oral rivaroxaban with dose-adjusted warfarin for the prevention of stroke and systemic embolism in patients with nonvalvular AF (defined as patients without rheumatic mitral valve disease, prosthetic heart valve or valve repair) who were at a moderate to high risk of stroke. Patients with AF and hemodynamically significant mitral stenosis or any valve prostheses were excluded [Patel et al. 2011]. The patient population (14,264) was randomized to receive fixed-dose rivaroxaban [20 mg once daily, or 15 mg once daily in patients with moderate renal impairment (CrCl 30–49 ml/min)] or dose-adjusted warfarin (target international normalized ratio 2.0–3.0) for the duration of the trial. The primary efficacy endpoint was the composite of stroke (ischemic or hemorrhagic) and systemic embolism. The principal safety outcome was a composite of major and nonmajor clinically relevant bleeding events.
The primary endpoint occurred in 188 patients in the rivaroxaban group (1.7% per year) and in 241 patients in the warfarin group (2.2% per year). In patients with nonvalvular AF, rivaroxaban was noninferior to warfarin for the prevention of stroke and systemic embolism (p < 0.001). Major and nonmajor clinically relevant bleeding occurred in 1475 patients in the rivaroxaban group (14.9% per year) and in 1449 patients in the warfarin group (14.5% per year). There were significant reductions in intracranial hemorrhage (0.5% versus 0.7%, p = 0.02) and fatal bleeding (0.2% versus 0.5%, p = 0.003) in the rivaroxaban group. Patients in the rivaroxaban group experienced more mucosal bleeding events, which included significantly more gastrointestinal and rectal bleeding (3.2% versus 2.2%; p < 0.001) and hematuria compared with the warfarin group. Liver safety parameters, as measured by elevated levels of ALT (>3× ULN) and serum bilirubin (>2× ULN), were similar between the rivaroxaban and warfarin groups (0.47% versus 0.50%, respectively).
ROCKET AF permitted the enrolment of patients with moderate renal impairment (CrCl 30–49 ml/min). Although these patients received a lower dose of rivaroxaban (15 mg once daily), rivaroxaban was also shown to be noninferior to warfarin in efficacy and safety analyses [Fox et al. 2011]. This finding suggests that, in patients who have moderate renal impairment, such as older patients, dose adjustment of rivaroxaban was effective for the prevention of stroke and systemic embolism [Fox et al. 2011].
Secondary prevention of acute coronary syndrome
Dual-antiplatelet therapy with ASA and a thienopyridine is standard treatment in ACS for secondary prevention of coronary events. However, treatment with clopidogrel and ASA in patients with ACS is associated with a 9–12% risk of death from cardiovascular causes, nonfatal myocardial infarction (MI) or stroke [Roe and Ohman, 2012; Wallentin et al. 2009; Wiviott et al. 2007; Yusuf et al. 2001]. In a meta-analysis of randomized trials investigating the effects of the addition of warfarin to ASA, the risk of major adverse events was not affected compared with ASA alone; however, there was a significant increase in the risk of major bleeding [Andreotti et al. 2006]. Furthermore, combination therapy with warfarin, ASA, and clopidogrel has been shown to increase the risk of bleeding after coronary stenting [Khurram et al. 2006]. There remains a need for treatment that is effective in reducing mortality in this patient group.
The efficacy and safety profile of rivaroxaban for the secondary prevention of ACS was investigated in a dose-escalation study (ATLAS ACS TIMI 46). Rivaroxaban was associated with a trend towards a reduction in the primary efficacy endpoint of death, MI, stroke, or severe recurrent ischemia requiring revascularization. For the principal safety outcome of clinically significant bleeding, a dose-dependent increase was observed with 5–20 mg rivaroxaban (once or twice daily) [Mega et al. 2009]. Two rivaroxaban regimens (2.5 mg and 5 mg twice daily) were chosen for further investigation, which were notably lower than the rivaroxaban doses used in clinical trials in other indications. ATLAS ACS 2 TIMI 51 was a phase III randomized, placebo-controlled multicenter, event-driven study designed to evaluate the efficacy and safety of rivaroxaban, which involved 15,526 patients [Mega et al. 2012]. Eligible patients presenting with ACS symptoms and who were diagnosed with ST-elevation MI, non-ST-elevation MI, or unstable angina. Patients were stabilized and enrolled from 24 h up to 7 days after hospital admission. Patients were stratified by the investigator’s intention to administer a thienopyridine (stratum 2) or not (stratum 1) at the time of enrollment, and were randomized to receive rivaroxaban (2.5 mg or 5 mg twice daily) or placebo. The primary efficacy endpoint was a composite of death from cardiovascular causes, MI, or stroke in patients with ACS. The mean duration of therapy was 13 months. Overall, there was a significant reduction in the rate of death in the combined rivaroxaban group (pooled data from the 2.5 mg and 5 mg twice daily doses) compared with placebo (8.9% versus 10.7%, p = 0.008). The secondary composite efficacy endpoint of death from any cause, MI, or stroke was also lower in the combined rivaroxaban group compared with patients receiving placebo (9.2% versus 11.0%, p = 0.006). The risk of stent thrombosis was reduced in the combined rivaroxaban group compared with the placebo group (2.3% versus 2.9%, p = 0.02). Both the 2.5 mg and 5 mg twice daily doses were significantly more effective in reducing death from cardiovascular causes, MI, or stroke compared with placebo. However, the lower dose of 2.5 mg twice daily was significantly more effective than the 5 mg twice daily dose in the reduction of rates of cardiovascular death (relative reduction 34%) and death from any cause (relative reduction 32%) compared with placebo.
The principal safety outcome was thrombolysis in MI (TIMI) major bleeding not related to coronary artery bypass grafting and was significantly higher in the rivaroxaban group (2.5 mg and 5 mg twice daily combined) compared with the placebo group (2.1% versus 0.6%, p < 0.001) [Mega et al. 2012]. Rates of TIMI minor bleeding, TIMI bleeding requiring medical attention, and intracranial bleeding were significantly higher in the rivaroxaban group compared with placebo (1.3% versus 0.5%, p = 0.003; 14.5% versus 7.5%, p < 0.001; 0.6% versus 0.2%, p = 0.009, respectively). Rates of TIMI major bleeding were also significantly higher for each of the 2.5 mg and 5 mg doses of rivaroxaban compared with placebo (p < 0.001 for both groups). However, levels of TIMI major bleeding were lower with the 2.5 mg twice daily dose of rivaroxaban compared with 5 mg twice daily (1.8% versus 2.4%, respectively; p = 0.12). The 2.5 mg twice daily dose was also associated with significantly lower rates of bleeding across all categories compared with rivaroxaban 5 mg twice daily. Rates of fatal bleeding were similar between the rivaroxaban and placebo groups (0.3% versus 0.2%, p = 0.66). Liver safety tests showed that rivaroxaban was well tolerated; elevated levels of plasma ALT (>3× ULN) and serum bilirubin (>2× ULN) were similar between treatment groups (0.2% for both groups) [Mega et al. 2012].
Future perspectives and practical considerations relating to use of rivaroxaban for hematologists
The completion of phase III clinical studies involving dabigatran and rivaroxaban, and their approval for use in the EU, Canada, and the USA, has increased the therapeutic options available to physicians. Utilization of factor Xa or thrombin inhibitors in clinical practice will involve multidisciplinary teams including orthopedic surgeons, cardiologists, internal medicine specialists, and hematologists. The need to implement effective anticoagulant treatment in patients with thromboembolic disease is likely to become more common with growing older populations and will, therefore, present new challenges to physicians. For example, effective anticoagulation treatment in patients already receiving therapy for other conditions or those with other underlying comorbidities will need to be considered. New guidelines from the ACCP recommending use of the newer oral anticoagulants may help physicians to define optimal thromboprophylaxis strategies [Guyatt et al. 2012]. In the future, new strategies to increase anticoagulant use in everyday practice, such as increased education of front-line health providers will be needed [Lloyd et al. 2012].
Results from clinical studies show that dose adjustment of rivaroxaban is not required based on age, hepatic impairment (not associated with coagulopathy), or sex [Bayer Pharma AG, 2011]; these results have provided efficacy and safety information on patients taking comedications such as nonsteroidal anti-inflammatory drugs, ASA, and platelet aggregation inhibitors [Fox et al. 2011; Kubitza et al. 2006, 2007].
Clinical trials involving rivaroxaban have included patients with mild or moderate renal impairment (CrCl 50–80 and CrCl 30–49 ml/min, respectively), but patients with severe renal impairment (CrCl 15–29 ml/min) were excluded from these trials. Elimination of rivaroxaban occurs in part via the kidneys and one consequence of renal impairment is to increase exposure to the drug via increased plasma concentrations. Renal clearance of rivaroxaban was shown to decrease with increasing renal impairment, and the area under the plasma concentration–time curve least-squares mean values were 1.44-fold, 1.52-fold, and 1.64-fold higher in patients with mild, moderate, and severe renal impairment, respectively, relative to healthy controls [Kubitza et al. 2010]. In a pooled analysis of subgroup data from the RECORD studies in patients with varying levels of renal function (CrCl >80, 50–80, <50 ml/min), renal function had no clinically relevant effect on the efficacy and safety of rivaroxaban (10 mg once daily) for the prevention of VTE after THR or TKR surgery [Turpie et al. 2011]. Similarly, in the EINSTEIN program, primary efficacy and safety outcomes were consistent in patients with mild and moderate renal impairment. In a subanalysis of the ROCKET AF study, the efficacy of rivaroxaban was similar in patients with AF with moderate renal impairment (CrCl 30–49 ml/min) who received dose adjustment (15 mg once daily) and this efficacy was consistent compared with those with normal renal function [Fox et al. 2011].
The newer oral anticoagulants do not require routine coagulation monitoring. In certain situations, it could be beneficial to monitor their effects in the case of overdose, emergency surgery, or hemorrhagic events [Lindhoff-Last et al. 2010; Samama and Guinet, 2011]. Rivaroxaban has been shown to have well defined effects in laboratory assessments; however, standardized tests are not widely available. Simple, reliable assays to assess rivaroxaban exposure can be useful in clinical situations such as suspected overdose, patients who require emergency surgery, patients with a thromboembolic or hemorrhagic event, or as a measure of compliance when noncompliance is suspected. Traditional clotting tests, including prothrombin time (PT), are not suitable for quantitative measurement of rivaroxaban exposure because the mechanism of action of rivaroxaban is different to that of traditional agents [Lindhoff-Last et al. 2010]. Moreover, the results of PT tests vary with different reagents, which cannot subsequently be standardized using the international normalized ratio system. However, using a standard curve based on the PT of plasma samples spiked with increasing concentrations of rivaroxaban, the anticoagulant effect of rivaroxaban can be expressed in µg/ml of plasma; a technique that could be used to monitor the pharmacodynamic effects of rivaroxaban in the clinic. Rivaroxaban also prolongs activated partial thromboplastin time in a concentration-dependent manner but only weakly, making it a less sensitive measure of rivaroxaban activity [Samama et al. 2010]. Anti-factor Xa chromogenic assays measuring factor Xa inhibition, when used with appropriate calibrators and controls, are thought to be specific and suitable for measurement of a wide range of rivaroxaban concentrations in plasma samples [Samama et al. 2012].
A risk of bleeding is associated with all anticoagulant use and remains challenging for physicians who need to balance the risk of bleeding with the benefits of anticoagulation. In the case of a patient who is bleeding, practical approaches include cessation of anticoagulant therapy, administration of prohemostatic agents, addressing the cause of bleeding, dialysis, or plasmapheresis (dabigatran only) [Crowther and Warkentin, 2008]. The need for reversal agents is less critical with the newer anticoagulants, owing to their shorter half lives. However, there may be situations, such as emergency surgery cases, in which agents may be required to reverse anticoagulant activity. A study by Eerenberg and colleagues showed that a four-factor prothrombin concentrate (which is not currently approved in the USA) was effective in the reversal of rivaroxaban activity in healthy volunteers [Eerenberg et al. 2011]. Several other preclinical studies show promise for a number of agents in the reversal of anticoagulant activity [Chan et al. 2011; Morishima et al. 2011; Zhou et al. 2011].
Summary
Thromboembolic disorders are major causes of morbidity and mortality. The efficacy and safety of rivaroxaban, as demonstrated to date in phase III clinical trials, will provide physicians with new therapeutic options. Rivaroxaban, together with the other newer oral anticoagulants, is likely to become the cornerstone of thrombosis management for a wide spectrum of patients.
Acknowledgments
The author would like to acknowledge Kelly Farrell, who provided editorial support with funding from Bayer HealthCare Pharmaceuticals and Janssen Research & Development, LLC.
Footnotes
Funding: This project received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Dr Turpie has been a consultant to Bayer HealthCare, Janssen Research & Development, LLC, Astellas, Portola, and Takeda.
References
- Agnelli G., Gallus A., Goldhaber S., Haas S., Huisman M., Hull R., et al. (2007) Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT (Oral Direct Factor Xa Inhibitor BAY 59-7939 in Patients with Acute Symptomatic Deep-Vein Thrombosis) study. Circulation 116: 180–187 [DOI] [PubMed] [Google Scholar]
- Andreotti F., Testa L., Biondi-Zoccai G., Crea F. (2006) Aspirin plus warfarin compared to aspirin alone after acute coronary syndromes: an updated and comprehensive meta-analysis of 25,307 patients. Eur Heart J 27: 519–526 [DOI] [PubMed] [Google Scholar]
- Ansell J., Hirsh J., Hylek E., Jacobson A., Crowther M., Palareti G. (2008) Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 133: 160S–198S [DOI] [PubMed] [Google Scholar]
- Bayer Pharma AG. (2011) Xarelto ® (rivaroxaban) Summary of Product Characteristics. Available at: http://www.xarelto.com/html/downloads/Xarelto_Summary_of_Product_Characteristics_Dec2011.pdf (accessed 4 May 2012).
- Bergqvist D., Benoni G., Bjorgell O., Fredin H., Hedlundh U., Nicolas S., et al. (1996) Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med 335: 696–700 [DOI] [PubMed] [Google Scholar]
- Büller H., Lensing A., Prins M., Agnelli G., Cohen A., Gallus A., et al. (2008) A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis. The EINSTEIN-DVT Dose-Ranging Study. Blood 112: 2242–2247 [DOI] [PubMed] [Google Scholar]
- Camm A., Kirchhof P., Lip G., Schotten U., Savelieva I., Ernst S., et al. (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 31: 2369–2429 [DOI] [PubMed] [Google Scholar]
- Chan H., Atkinson H., Goncharenko M., Berry L., Chan A. (2011) Reversal of dabigatran using recombinant activated factor VII and activated prothrombin complex concentrates in thromboelastography assay. J Thromb Haemost 9: 576–577 Abstract P-WE-180. [Google Scholar]
- Cohen A., Agnelli G., Anderson F., Arcelus J., Bergqvist D., Brecht J., et al. (2007) Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost 98: 756–764 [DOI] [PubMed] [Google Scholar]
- Cohen A., Spiro T., Büller H., Haskell L., Hu D., Hull R., et al. (2011a) Extended-duration rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. J Thromb Thrombolysis 31: 407–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Spiro T., Büller H., Haskell L., Hu D., Hull R., et al. (2011b) Rivaroxaban compared with enoxaparin for the prevention of venous thromboembolism in acutely ill medical patients. Available at: http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_425442.pdf (accessed 25 April 2012).
- Connolly S., Ezekowitz M., Yusuf S., Eikelboom J., Oldgren J., Parekh A., et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139–1151 [DOI] [PubMed] [Google Scholar]
- Crowther M., Warkentin T. (2008) Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: focus on new anticoagulant agents. Blood 111: 4871–4879 [DOI] [PubMed] [Google Scholar]
- Deitelzweig S., Johnson B., Lin J., Schulman K. (2011) Prevalence of clinical venous thromboembolism in the USA: Current trends and future projections. Am J Hematol 86: 217–220 [DOI] [PubMed] [Google Scholar]
- Eerenberg E., Kamphuisen P., Sijpkens M., Meijers J., Buller H., Levi M. (2011) Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 124: 1573–1579 [DOI] [PubMed] [Google Scholar]
- Eriksson B., Borris L., Dahl O., Haas S., Huisman M., Kakkar A., et al. (2006) A once-daily, oral, direct factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement. Circulation 114: 2374–2381 [DOI] [PubMed] [Google Scholar]
- Eriksson B., Borris L., Friedman R., Haas S., Huisman M., Kakkar A., et al. (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 358: 2765–2775 [DOI] [PubMed] [Google Scholar]
- Eriksson B., Dahl O., Rosencher N., Kurth A., van Dijk C., Frostick S., et al. (2007a) Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 5: 2178–2185 [DOI] [PubMed] [Google Scholar]
- Eriksson B., Dahl O., Rosencher N., Kurth A., van Dijk C., Frostick S., et al. (2007b) Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 370: 949–956 [DOI] [PubMed] [Google Scholar]
- Eriksson B., Kakkar A., Turpie A., Gent M., Bandel T., Homering M., et al. (2009) Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J Bone Joint Surg Br 91: 636–644 [DOI] [PubMed] [Google Scholar]
- Fox K., Piccini J., Wojdyla D., Becker R., Halperin J., Nessel C., et al. (2011) Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 32: 2387–2394 [DOI] [PubMed] [Google Scholar]
- Geerts W., Bergqvist D., Pineo G., Heit J., Samama C., Lassen M., et al. (2008) Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 133: 381S–453S [DOI] [PubMed] [Google Scholar]
- Geerts W., Heit J., Clagett G., Pineo G., Colwell C., Anderson F., et al. (2001) Prevention of venous thromboembolism. Chest 119: 132S–175S [DOI] [PubMed] [Google Scholar]
- Goldhaber S., Leizorovicz A., Kakkar A., Haas S., Merli G., Knabb R., et al. (2011) Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med 365: 2167–2177 [DOI] [PubMed] [Google Scholar]
- Guyatt G., Akl E., Crowther M., Gutterman D., Schünemann H. and American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel (2012) Executive summary: antithrombotic therapy and prevention, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141: 7S–47S [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder S., Graff J., Hentig N., Misselwitz F., Kubitza D., Zuehlsdorf M., et al. (2004) Effects of BAY 59-7939, an oral, direct factor Xa inhibitor, on thrombin generation in healthy volunteers. Pathophysiol Haemost Thromb 33(Suppl. 2): abstract PO078 [Google Scholar]
- Heit J., Cohen A., Anderson F. and on behalf of the VTE Impact Assessment Group (2005) Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. Blood (ASH Annual Meeting Abstracts) 106: abstract 910. [Google Scholar]
- Hirsh J., Bauer K., Donati M., Gould M., Samama M., Weitz J., et al. (2008) Parenteral anticoagulants: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 133: 141S–159S [DOI] [PubMed] [Google Scholar]
- Hull R., Schellong S., Tapson V., Monreal M., Samama M., Nicol P., et al. (2010) Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med 153: 8–18 [DOI] [PubMed] [Google Scholar]
- Huo M., Spyropoulos A. (2011) The eighth American College of Chest Physicians guidelines on venous thromboembolism prevention: implications for hospital prophylaxis strategies. J Thromb Thrombolysis 31: 196–208 [DOI] [PubMed] [Google Scholar]
- Kakkar A., Brenner B., Dahl O., Eriksson B., Mouret P., Muntz J., et al. (2008) Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 372: 31–39 [DOI] [PubMed] [Google Scholar]
- Kearon C., Kahn S., Agnelli G., Goldhaber S., Raskob G., Comerota A., et al. (2008) Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 133: 454S–545S [DOI] [PubMed] [Google Scholar]
- Khurram Z., Chou E., Minutello R., Bergman G., Parikh M., Naidu S., et al. (2006) Combination therapy with aspirin, clopidogrel and warfarin following coronary stenting is associated with a significant risk of bleeding. J Invasive Cardiol 18: 162–164 [PubMed] [Google Scholar]
- Kubitza D., Becka M., Mueck W., Halabi A., Maatouk H., Klause N., et al. (2010) Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban – an oral, direct factor Xa inhibitor. Br J Clin Pharmacol 70: 703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitza D., Becka M., Mueck W., Zuehlsdorf M. (2006) Safety, tolerability, pharmacodynamics, and pharmacokinetics of rivaroxaban – an oral, direct factor Xa inhibitor – are not affected by aspirin. J Clin Pharmacol 46: 981–990 [DOI] [PubMed] [Google Scholar]
- Kubitza D., Becka M., Mueck W., Zuehlsdorf M. (2007) Rivaroxaban (BAY 59-7939) – an oral, direct factor Xa inhibitor – has no clinically relevant interaction with naproxen. Br J Clin Pharmacol 63: 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitza D., Becka M., Roth A., Mueck W. (2008) Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin 24: 2757–2765 [DOI] [PubMed] [Google Scholar]
- Kubitza D., Becka M., Voith B., Zuehlsdorf M. (2005a) Effect of enoxaparin on the safety, tolerability, pharmacodynamics and pharmacokinetics of BAY 59-7939 – an oral, direct factor Xa inhibitor. J Thromb Haemost 3(Suppl. 1): abstract P1704. [Google Scholar]
- Kubitza D., Becka M., Voith B., Zuehlsdorf M., Wensing G. (2005b) Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther 78: 412–421 [DOI] [PubMed] [Google Scholar]
- Kubitza D., Becka M., Wensing G., Voith B., Zuehlsdorf M. (2005c) Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939 – an oral, direct factor Xa inhibitor – after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 61: 873–880 [DOI] [PubMed] [Google Scholar]
- Lassen M., Ageno W., Borris L., Lieberman J., Rosencher N., Bandel T., et al. (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 358: 2776–2786 [DOI] [PubMed] [Google Scholar]
- Lassen M., Gallus A., Raskob G., Pineo G., Chen D., Ramirez L., et al. (2010a) Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 363: 2487–2498 [DOI] [PubMed] [Google Scholar]
- Lassen M., Raskob G., Gallus A., Pineo G., Chen D., Hornick P., et al. (2010b) Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 375: 807–815 [DOI] [PubMed] [Google Scholar]
- Leizorovicz A., Mismetti P. (2004) Preventing venous thromboembolism in medical patients. Circulation 110: IV13–IV19 [DOI] [PubMed] [Google Scholar]
- Lemmens R., Hermans S., Nuyens D., Thijs V. (2011) Genetics of atrial fibrillation and possible implications for ischemic stroke. Stroke Res Treat 2011: 208694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhoff-Last E., Samama M., Ortel T., Weitz J., Spiro T. (2010) Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit 32: 673–679 [DOI] [PubMed] [Google Scholar]
- Lloyd N., Douketis J., Cheng J., Schunemann H., Cook D., Thabane L., et al. (2012) Barriers and potential solutions toward optimal prophylaxis against deep vein thrombosis for hospitalized medical patients: a survey of healthcare professionals. J Hosp Med 7: 28–34 [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D., Adams R., Brown T., Carnethon M., Dai S., De Simone G., et al. (2010) Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation 121: e46–e215 [DOI] [PubMed] [Google Scholar]
- Mega J., Braunwald E., Mohanavelu S., Burton P., Poulter R., Misselwitz F., et al. (2009) Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet 374: 29–38 [DOI] [PubMed] [Google Scholar]
- Mega J., Braunwald E., Wiviott S., Bassand J., Bhatt D., Bode C., et al. (2012) Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 366: 9–19 [DOI] [PubMed] [Google Scholar]
- Morishima Y., Honda Y., Kamisato C., Furugohri T., Shibano T. (2011) Reversal agents for edoxaban, a factor Xa inhibitor: effects of factor VIIa, anti-inhibitor coagulant complex, prothrombin complex concentrate, fresh plasma, and vitamin K. J Thromb Haemost 9: 135 Abstract P-MO-246. [Google Scholar]
- Murray C., Lopez A. (1997) Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 349: 1498–1504 [DOI] [PubMed] [Google Scholar]
- Nutescu E. (2003) Antithrombotic therapy for the treatment of venous thromboembolism. Am J Manag Care 9: S103–S114 [PubMed] [Google Scholar]
- Patel M., Mahaffey K., Garg J., Pan G., Singer D., Hacke W., et al. (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365: 883–891 [DOI] [PubMed] [Google Scholar]
- Payne D., Hayes P., Jones C., Belham P., Naylor A., Goodall A. (2002) Combined therapy with clopidogrel and aspirin significantly increases the bleeding time through a synergistic antiplatelet action. J Vasc Surg 35: 1204–1209 [DOI] [PubMed] [Google Scholar]
- Perzborn E., Strassburger J., Wilmen A., Pohlmann J., Roehrig S., Schlemmer K., et al. (2005) In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939 − an oral, direct factor Xa inhibitor. J Thromb Haemost 3: 514–521 [DOI] [PubMed] [Google Scholar]
- Prandoni P., Noventa F., Ghirarduzzi A., Pengo V., Bernardi E., Pesavento R., et al. (2007) The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 92: 199–205 [DOI] [PubMed] [Google Scholar]
- Rayner M., Allender S., Scarborough P. (2009) Cardiovascular disease in Europe. Eur J Cardiovasc Prev Rehabil 16(Suppl. 2): S43–S47 [DOI] [PubMed] [Google Scholar]
- Rodger M., Kahn S., Wells P., Anderson D., Chagnon I., Le Gal G., et al. (2008) Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ 179: 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe M., Ohman E. (2012) A new era in secondary prevention after acute coronary syndrome. N Engl J Med 366: 85–87 [DOI] [PubMed] [Google Scholar]
- Roger V., Go A., Lloyd-Jones D., Adams R., Berry J., Brown T., et al. (2011) Heart disease and stroke statistics – 2011 update: a report from the American Heart Association. Circulation 123: e18–e209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samama M. (2011) The mechanism of action of rivaroxaban – an oral, direct factor Xa inhibitor – compared with other anticoagulants. Thromb Res 127: 497–504 [DOI] [PubMed] [Google Scholar]
- Samama M., Contant G., Spiro T., Perzborn E., Guinet C., Gourmelin Y., et al. (2012) Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost 107: 379–387 [DOI] [PubMed] [Google Scholar]
- Samama M., Guinet C. (2011) Laboratory assessment of new anticoagulants. Clin Chem Lab Med 49: 761–772 [DOI] [PubMed] [Google Scholar]
- Samama M., Martinoli J., Le Flem L., Guinet C., Plu-Bureau G., Depasse F., et al. (2010) Assessment of laboratory assays to measure rivaroxaban – an oral, direct factor Xa inhibitor. Thromb Haemost 103: 815–825 [DOI] [PubMed] [Google Scholar]
- The EINSTEIN Investigators (2010) Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 363: 2499–2510 [DOI] [PubMed] [Google Scholar]
- The EINSTEIN–PE Investigators (2012) Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 366: 1287–1297 [DOI] [PubMed] [Google Scholar]
- Turpie A., Lassen M., Davidson B., Bauer K., Gent M., Kwong L., et al. (2009) Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 373: 1673–1680 [DOI] [PubMed] [Google Scholar]
- Turpie A., Lassen M., Eriksson B., Gent M., Berkowitz S., Misselwitz F., et al. (2011) Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Pooled analysis of four studies. Thromb Haemost 105: 444–453 [DOI] [PubMed] [Google Scholar]
- Wallentin L., Becker R., Budaj A., Cannon C., Emanuelsson H., Held C., et al. (2009) Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361: 1045–1057 [DOI] [PubMed] [Google Scholar]
- Weitz J., Hirsh J., Samama M. (2008) New antithrombotic drugs: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 133: 234S–256S [DOI] [PubMed] [Google Scholar]
- Wiviott S., Braunwald E., McCabe C., Montalescot G., Ruzyllo W., Gottlieb S., et al. (2007) Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 357: 2001–2015 [DOI] [PubMed] [Google Scholar]
- Wolf P., Abbott R., Kannel W. (1991) Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22: 983–988 [DOI] [PubMed] [Google Scholar]
- Yusuf S., Zhao F., Mehta S., Chrolavicius S., Tognoni G., Fox K. (2001) Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 345: 494–502 [DOI] [PubMed] [Google Scholar]
- Zhou W., Schwarting S., Illanes S., Liesz A., Middelhoff M., Zorn M., et al. (2011) Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke 42: 3594–3599 [DOI] [PubMed] [Google Scholar]


