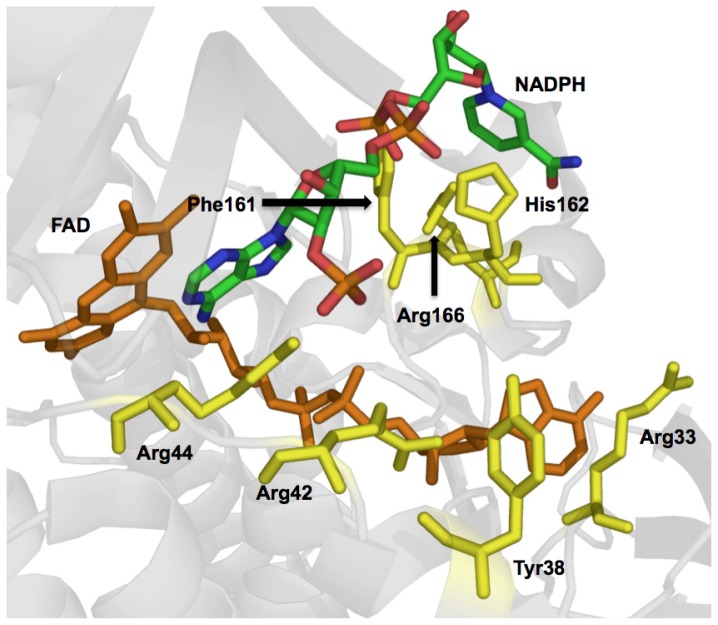
Figure 7.
The Structure of PHBH Variant, Arg220Gln with a Molecule of NADPH Bound (PDB ID:1KOJ), and the Positions of Residues Implicated in NADPH Binding in this and Other Studies. In the structure the adenine of NADPH is positioned in a stacking orientation with the isoalloxazine ring and the nicotinamide ring is oriented above the binding cleft between Phe161 and His162. It is important to note that in this binding mode that only two direct hydrogen-bond contacts are made to NADPH. In the rationalization of the structure it had been proposed that when hydride transfer is made, NAPDH forms a hooked conformation to orient the nicotinamide in close proximity to the N(5) flavin site [72]. In other studies a range of different residues that roughly track the binding cleft of the FAD have been implicated by mutagenesis as contributors to both NADPH binding and NADPH/NADH specificity. These residues include Arg33, Tyr38, Arg42, Arg44, Phe161, and Arg166 [92,115,116].