Abstract
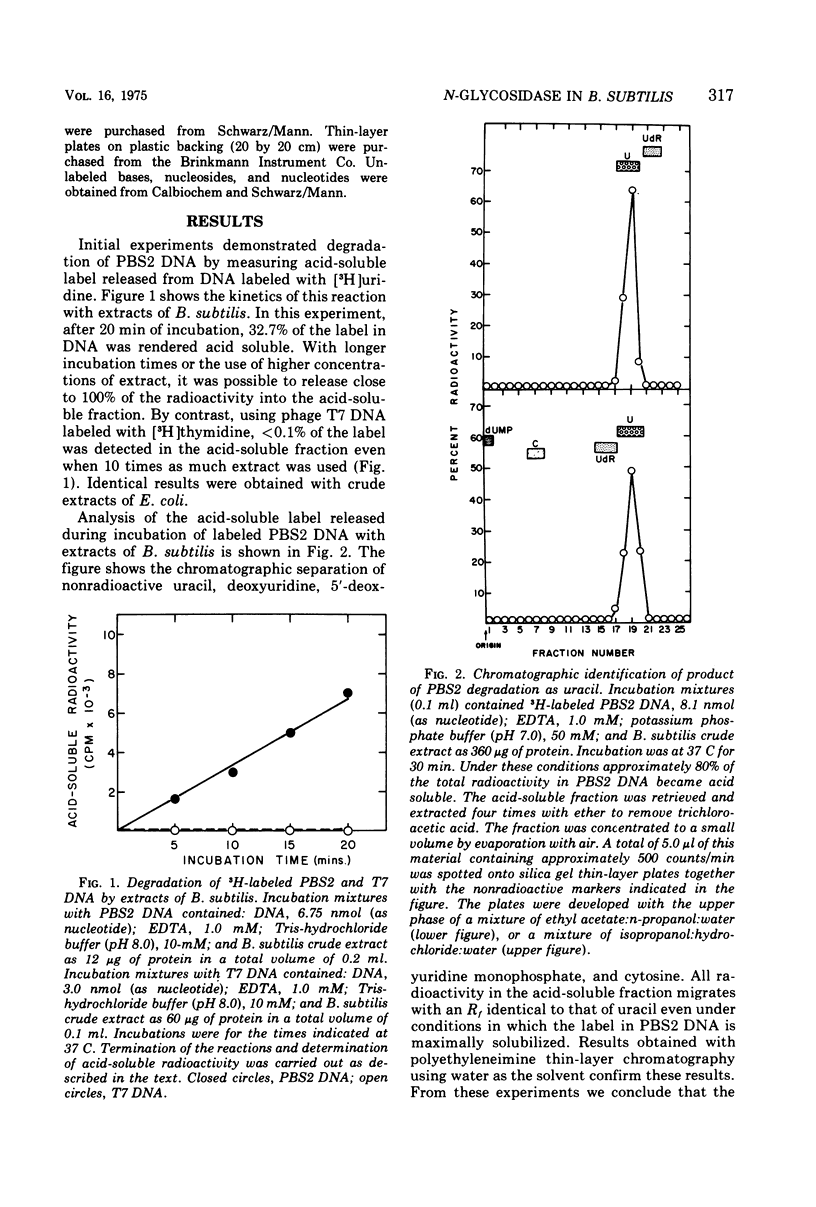
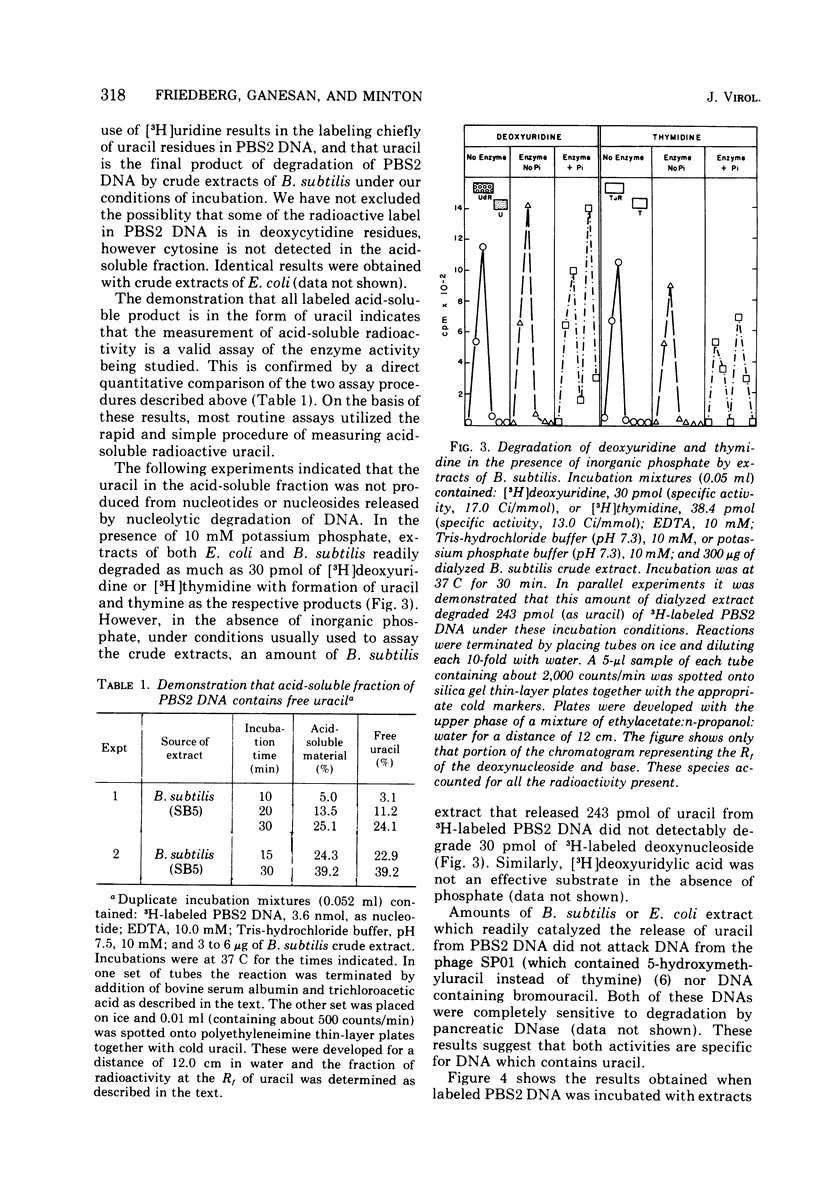
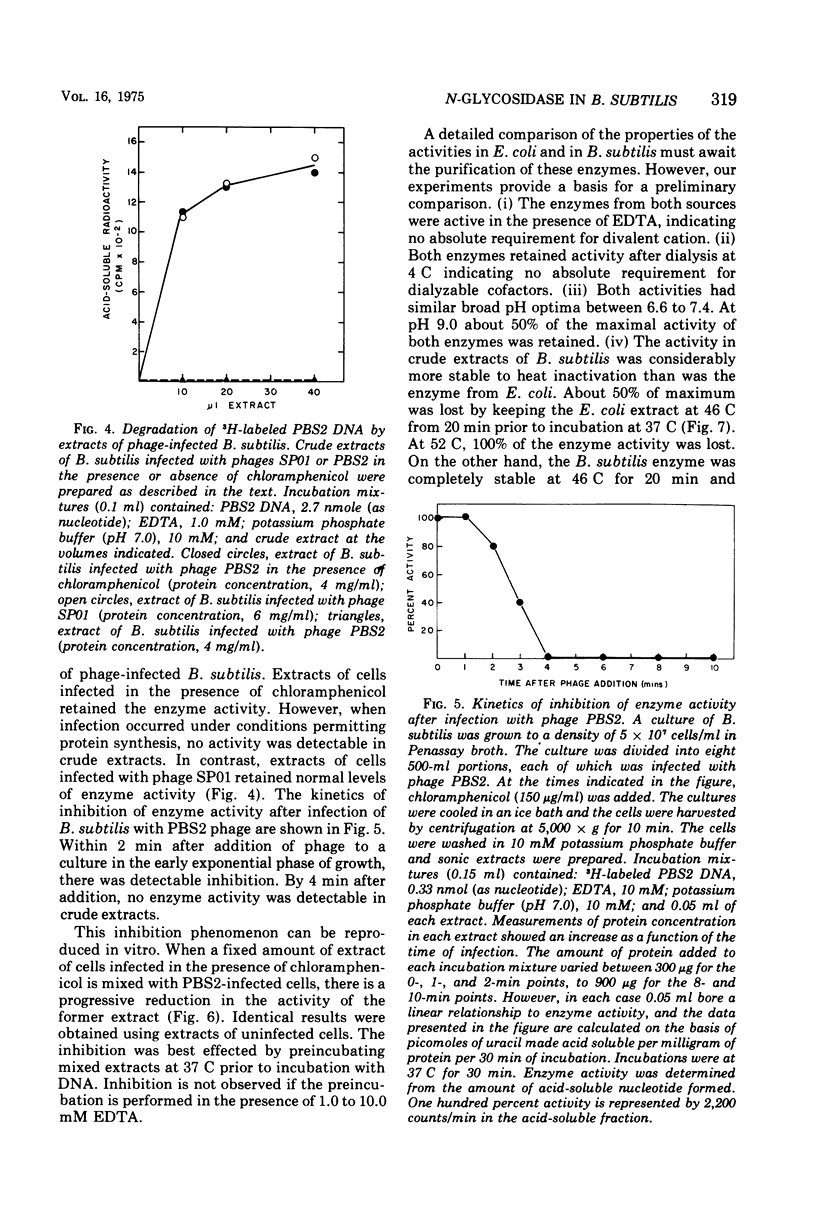
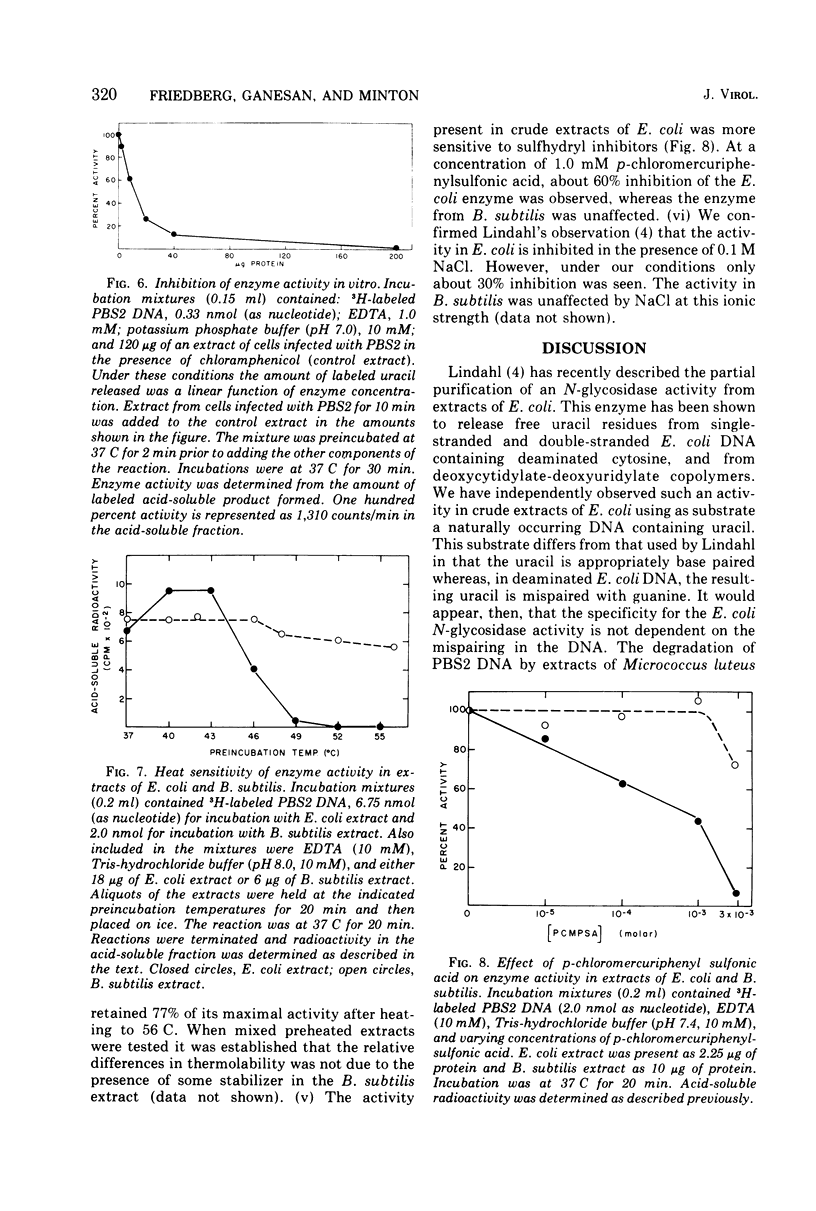
We have detected in crude extracts of Bacillus subtilis an N-glycosidase activity which catalyzes the release of free uracil from DNA of the subtilis phage PBS2 labeled with [3H]uridine. This DNA contains deoxyuridine instead of thymidine. The enzyme is active in the presence of 1.0 mM EDTA and under these conditions Escherichia coli or T7 DNA labeled with [3H]thymidine is not degraded to labeled acid-soluble products. The activity resembles an N-glycosidase from E. coli which releases free uracil from DNA containing deaminated cytosine residues. Both enzymes in crude extracts are active in the presence of EDTA, do not require dialyzable co-factors, and have the same pH optimum. They differ in that the enzyme from E. coli is more sensitive to heat, sulfhydryl reagents, and salt. The enzyme from B. subtilis is inactive on DNA containing 5-bromouracil or hydroxymethyluracil. Extracts of PBS2-infected B. subtilis lose the N-glycosidase activity within 4 min after infection and contain a factor that inhibits the N-glycosidase activity within 4 min after infection and contain a factor that inhibits the N-glycosidase activity in extracts of uninfected cells in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann K., Friedberg E. C. Measurement of thymine dimers in DNA by thin-layer chromatography. Anal Biochem. 1973 May;53(1):124–131. doi: 10.1016/0003-2697(73)90413-2. [DOI] [PubMed] [Google Scholar]
- Hunter B. I., Yamagishi H., Takahashi I. Molecular weight of bacteriophage PBS 1 deoxyribonucleic acid. J Virol. 1967 Aug;1(4):841–842. doi: 10.1128/jvi.1.4.841-842.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T. An N-glycosidase from Escherichia coli that releases free uracil from DNA containing deaminated cytosine residues. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3649–3653. doi: 10.1073/pnas.71.9.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Price A. R., Cook S. J. New deoxyribonucleic acid polymerase induced by Bacillus subtilis bacteriophage PBS2. J Virol. 1972 Apr;9(4):602–610. doi: 10.1128/jvi.9.4.602-610.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI I., MARMUR J. Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis. Nature. 1963 Feb 23;197:794–795. doi: 10.1038/197794a0. [DOI] [PubMed] [Google Scholar]



