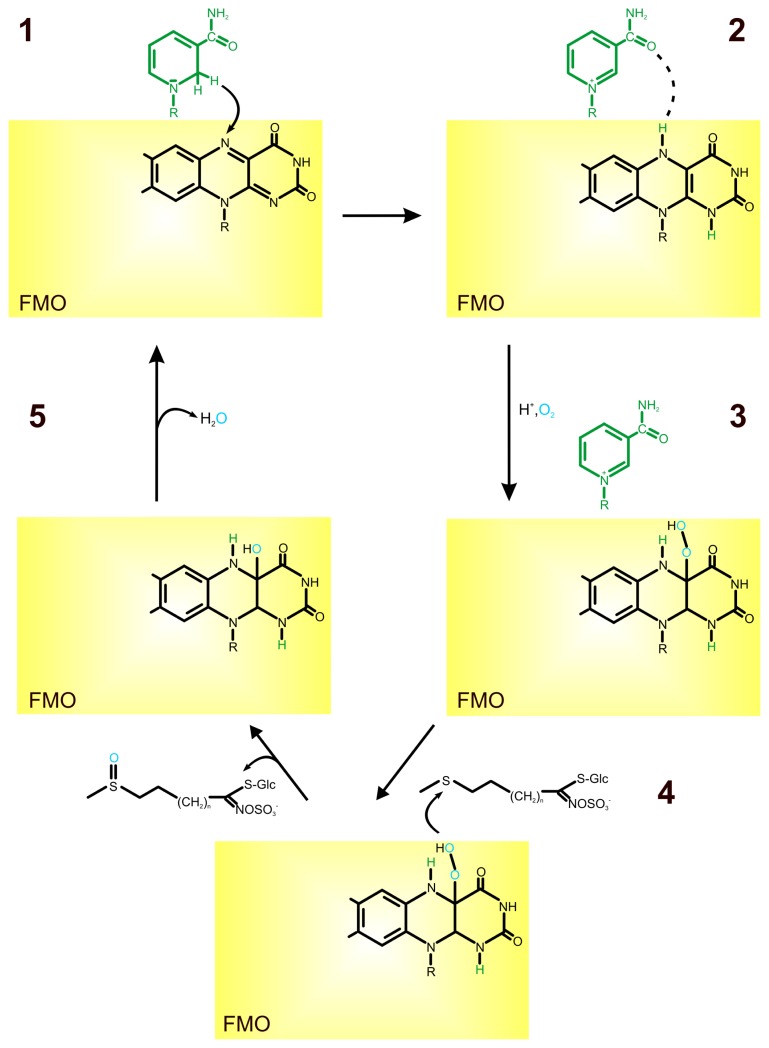
Figure 1.
Role of FAD in S-oxygenation of glucosinolate. FAD is reduced to FADH2 through a hydride ion transfer from NADPH (1, 2); FADH2 binds molecular oxygen at the 4α position of the isoalloxazine ring resulting in a 4α-hydroperoxyflavin adenine dinucleotide (FAD-OOH) formation (3); when a suitable substrate, such as S in methylthioalkyl, binds to the protein/FAD-OOH complex it is oxygenated to SO through the OOH moiety (4); the oxygen atom reacts to form water, NADP is released and binding of another NADPH starts a new cycle (5) [11,18,19].