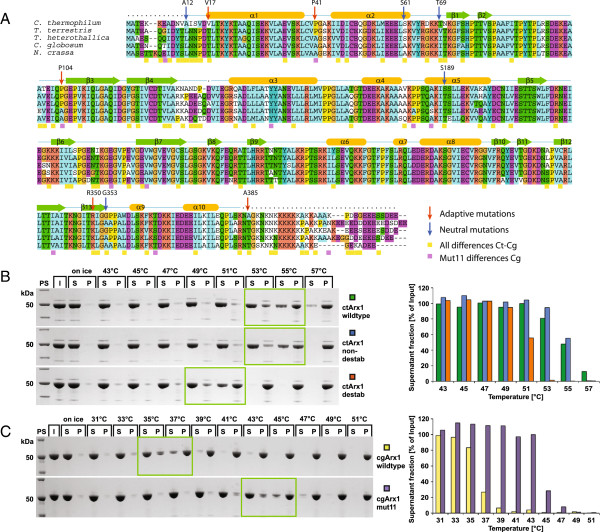
Figure 4.
Amino acids responsible for thermostability. A) Alignment of Arx1 protein of the three thermophilic Sordariomycetes, C. globosum and N. crassa. Arrows represent positions of introduced mutations that we predict to be adaptive to thermophily (red) or neutral (blue). Secondary structure elements are indicated above the alignment (cylinder: α-helix; arrow: β–strand; line: loop regions; dotted line: not solved in crystal structure). Violet squares represent mutations in the cgArx1mut11 and yellow squares represent all other differences between ctArx1 and cgArx1. Amino acids are colored with the default color scheme of ClustalX [38]. B) Arx1 from C. thermophilum (ctArx1) is thermostable and its thermostability can be influenced by mutating residues predicted to be adaptive for thermophily. Recombinant wildtype ctArx1 and mutant ctArx1-nondestab and ctArx1-destab proteins were affinity-purified and incubated at the indicated temperatures for one hour. Then the proteins were separated into supernatant (S) and pellet (P) fractions by centrifugation and subjected to SDS-PAGE and Coomassie stain in comparison to the input (I). PS, protein standard. The five mutations present in ctArx1-nondestab and ctArx1-destab were predicted to be neutral or adaptive mutations to thermophily. The fraction of protein present in the supernatant fraction was analyzed by quantifying the Coomassie stained protein bands with Aida Image Analyzer v. 4.00. The input was set to 100%. C) Recombinant cgArx1mut11 protein contains mutations in 11 residues predicted to have destabilized the protein of C. globosum. Thermostability analysis of recombinant cgArx1 and cgArgx1mut11 as described in (B).