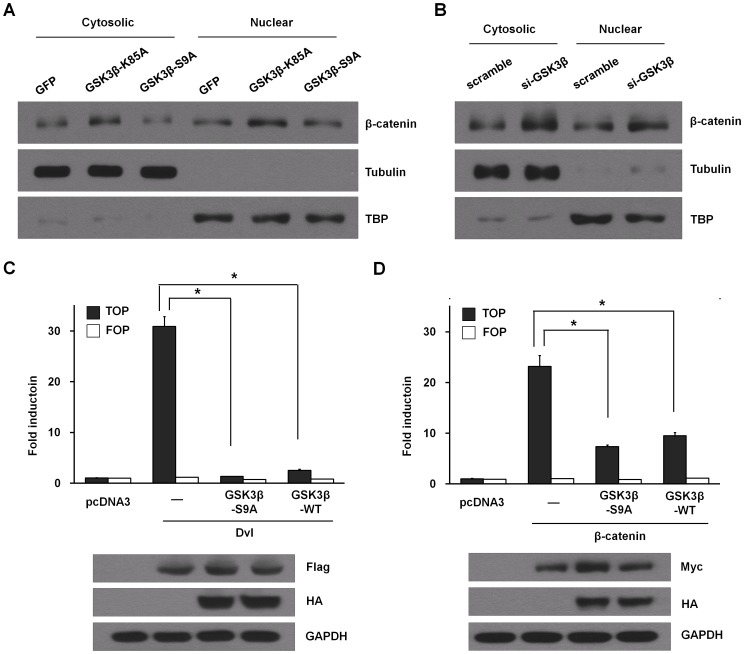
Figure 5. GSK3β activity regulates β-catenin level in ADSCs.
(A) ADSCs were infected with a retrovirus expressing GFP, GSK3β-K85A, or GSK3β-S9A for 24 hours and infected cells were selected with 2 μg/ml puromycin. After 48 hours, the cells were fractionated into nuclear and cytosolic fractions and immunoblotted using β-catenin antibody. The purity of fractions was confirmed with TATA-binding protein (TBP) and tubulin antibodies, respectively. (B) ADSCs were transiently transfected with 10 nM GSK3β siRNA or a nonrelevant siRNA (scramble). After 48 hours, cells were processed and analyzed as in (A). (C, D) HEK293T cells were transfected with a wild-type TCF/LEF reporter (TOPFLASH) and a mutant inactive form (FOPFLASH), together with the indicated constructs and promoter activity, was measured by luciferase assay 24 hours later. The luciferase activities were normalized for pRenilla, the control reporter, activity. Overexpression of Flag-tagged Dvl (C) or Myc-tagged β-catenin (D) together with HA-tagged GSK3β-S9A or WT was detected by immunoblotting. Data represent mean ± S.D. and are representative of at least 3 experiments. *p≤0.01, significantly different from mouse Dvl-Flag transfected group or mouse β-catenin-myc transfected group.