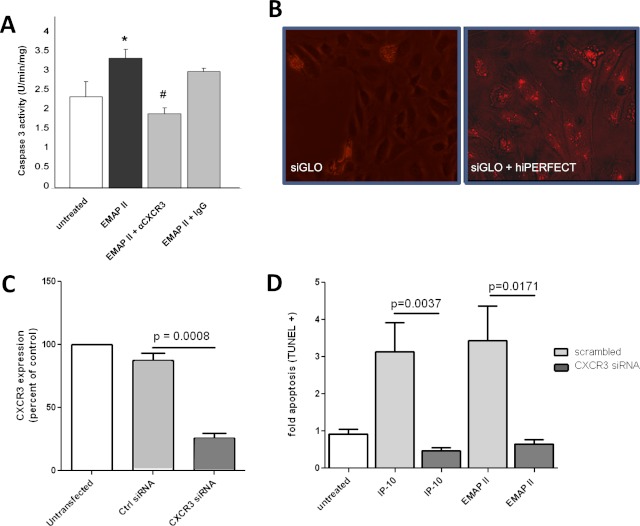
Figure 1.
Targeting the CXCR3 receptor abrogates endothelial monocyte–activating polypeptide II (EMAP II)–induced endothelial cell apoptosis. (A) Human lung microvascular endothelial cells (HLMVECs) were grown in low serum media with and without EMAP II (10 μg/ml for 16 hours) in the presence of a specific CXCR3 antibody (1 μg/ml; 30-minute pretreatment) or isotype control antibody (1 μg/ml). Caspase-3 activity assay (Promega, Fitchburg, WI) was performed to assess apoptosis. *P < 0.05, compared with untreated cells. #P < 0.01, compared with IgG control samples. (B) About 20,000 human umbilical vein endothelial cells (HUVECs) were grown in eight-well chamber slides to confluence, and were used to transfect cells transiently with hiPERFECT. Shown is a transfection-positive control sample with siGLO Red (10 nM), with and without transfection reagent in the absence of small interfering RNA (siRNA). (C) HUVECs (from B) were transfected with CXCR3 siRNA or scrambled control RNA, using hiPERFECT. CXCR3 expression was assessed 48 hours after transfection by using real-time PCR analysis, and normalized to eukaryotic initiation factor 1 alpha (EIF1α). (D) At 48 hours after transfection, interferon-inducible protein (IP)–10 (4 nM) or EMAP II (250 nM) was added and incubated for an additional 16 hours. The light gray bars represent cells treated with control siRNA, and dark gray bars represent cells treated with CXCR3 siRNA. dUTP nick end labeling (TUNEL) staining was performed to assess apoptosis in cells in situ, as quantitated by MetaMorph software.