Table 1.
Ligand Effects on Reductive Conjugate Addition.a
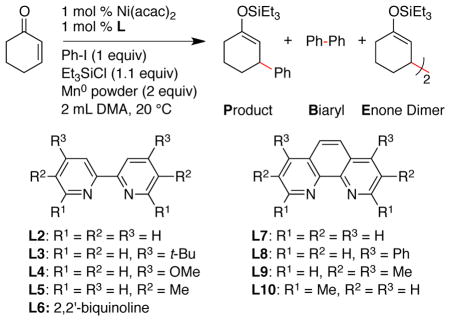
| ||||||
|---|---|---|---|---|---|---|
| entry | ligand (L) | t (h) | P (%) | B (%) | E (%) | PhH (%) |
| 1 | none | 24 | 28b | 6 | 19 | 1 |
| 2 | py (1 equiv) | 15 | 39c | 3 | 14 | 4 |
| 3d | L1 | 18 | 24 | 42 | 98 | 10 |
| 4 | L2 | 24 | 67 | 13 | 51 | 6 |
| 5 | L3 | 24 | 41c | 16 | 38 | 10 |
| 6 | L4 | 24 | 41e | 36 | 54 | 11 |
| 7 | L5 | 72 | 60 | 0 | 34 | 0 |
| 8 | L6 | 24 | 41c | 19 | 47 | 11 |
| 9 | L7 | 24 | 28c | 12 | 44 | 11 |
| 10 | L8 | 24 | 34f | 24 | 46 | 11 |
| 11 | L9 | 24 | 43e | 34 | 36 | 9 |
| 12 | L10 | 2 | 99 | 0 | 13 | 4 |
See Supporting Information for full experimental details. Yields for P, B, and Ph-H are corrected vs internal standard (dodecane). Yields of E are uncorrected.
>50% of both enone and PhI remained.
>10% of both enone and PhI remained.
Reaction run with 10 mol % [Ni] and L1 in DMF.
>10% of enone remained.
>10% of PhI remained.
