Table 4.
Stoichiometric Reactivity of Allylnickel IIA.a
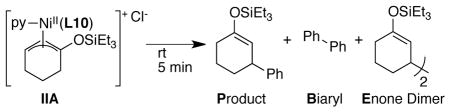
| ||||||
|---|---|---|---|---|---|---|
| entry | conditionsb | Yield P (%) |
Yield B (%) |
Yield E (%) |
||
| PhI | Mn0 | Et3SiCl | ||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 100 | 0 | 0 | 12 | 0 | 0 |
| 3 | 100 | 0 | 110 | 7 | 0 | 0 |
| 4 | 100 | 110 | 200 | 71 | 0 | 0 |
| 5 | 1.0 | 110 | 200 | 28 | 0 | 0 |
| 6 | 100 | 0d | 0 | 26 | 0 | 0 |
| 7 | catalytic reaction with Ni(acac)2 and L10 | 99e | 0 | 13e | ||
| 8 | Catalytic reaction with Ni(acac)2, L10, and pyr | 69e | 0 | 14e | ||
Nickel complexes were generated in situ at a concentration of 2.5 mM in DMA and reacted with the noted reagents. Analysis at 5 minutes (GC, corrected) provided the stated yields. For stoichiometric reactions (1–6), the yield is calculated from starting nickel complex IIA.
Equivalents with respect to [Ni].
Mn0 powder was pre-stirred with Et3SiCl.
TDAE = tetrakis(dimethylamino)ethylene.
Yield calculated from the amount of enone added to catalytic reactions (0.5 mmol).
