Abstract
Objective
To determine the effect of bovine lactoferrin on prevention of diarrhea in children.
Study design
We conducted a community-based randomized double-blind placebo controlled trial comparing supplementation with bovine lactoferrin versus placebo. Previously weaned children were enrolled at 12–18 months and followed for 6 months with daily home visits for data collection and supplement administration. Anthropometric measures were done monthly.
Results
555 children were randomized: 277 to lactoferrin and 278 to placebo; 65 dropped out; 147,894 doses were administered (92% compliance). Overall there were 91,446 child-days of observation and 1,235 diarrhea episodes lasting 6,219 days. The main pathogens isolated during diarrheal episodes were norovirus (35.0%), enteropathogenic E. coli (11.4%), Campylobacter (10.6%), enteroaggregative E. coli (8.4%), enterotoxigenic E. coli (6.9%) and Shigella (6.6%). The diarrhea incidence was not different between groups: 5.4 vs. 5.2 episodes/child/year for lactoferrin and placebo, respectively (p=0.375). However, the diarrhea longitudinal prevalence was lower in the lactoferrin group (6.6% vs. 7.0%, p=0.017) as well as the median duration of episodes (4.8 vs. 5.3 days, p=0.046), proportion of episodes with moderate or severe dehydration (1.0% vs. 2.6%, p=0.045) and liquid stools load (95.0 vs. 98.6) liquid stools/child/year, p<0.001). There were no adverse events related to the intervention.
Conclusions
Although there was no decrease in diarrhea incidence, longitudinal prevalence and severity were decreased with lactoferrin.
Keywords: lactoferrin, diarrhea, children, prevention, clinical trial
The WHO estimates 8.1 million deaths occur yearly in children (<5 years of age) with diarrhea accounting for 14% of deaths.1 In addition to causing mortality, diarrhea has serious long term effects with multiple episodes and persistent diarrhea affecting growth, nutrition and cognition.2 Breastfeeding is the most cost effective intervention for protecting children against diarrhea and all causes of mortality.3 Exclusive breast-feeding, and to a lesser extent partial breast-feeding, protects against acute and persistent diarrhea.4 Breastfeeding helps protect infants by serving as a source of nutrition uncontaminated by environmental pathogens. It is also generally assumed that protection is due to the multiple anti-infective, anti-inflammatory, and immunoregulatory factors transmitted through milk, including secretory antibodies, glycans, lactoferrin, leukocytes, cytokines and other components produced by the mother’s immune system.5,6
Lactoferrin, the second most abundant protein in human milk, is also found in most exocrine secretions including tears, saliva, intestinal mucus and genital secretions, and in the specific granules of neutrophils. Lactoferrin has multiple putative activities (anti-microbial, anti-inflammatory, immunomodulatory).7–9 It has been thought to protect against Gram negative enteropathogens by sequestration of iron essential for bacterial growth, binding to the lipid A portion of LPS on the cell surface, and disrupting the bacterial cell membrane.10,11 In vitro lactoferrin decreases virulence of enteropathogens by decreasing their ability to adhere to or invade mammalian cells, and by binding to, or degrading, specific virulence proteins.12–14 Human (hLF) and bovine lactoferrin (bLF) despite minor structural and biochemical differences have similar bioactivity, as assessed in vitro and in animal models.15,16 bLF has previously been shown to be safe in infants.17–19 Our hypothesis was that bLF would lower the frequency and severity of diarrhea in children related to its multiple anti-bacterial activities.12–14,20,21 The primary objectives were to determine the effects of lactoferrin on prevention of diarrhea episodes and on growth in previously weaned children.
METHODS
A community-based randomized double blind placebo-controlled trial was conducted in children from Lima, Peru, comparing twice daily supplementation with bLF versus placebo administered for 6 months with monitoring of diarrhea and growth. Eligible children were previously weaned 12–18 months old. Exclusion criteria were a history of severe, persistent or chronic diarrhea, severe malnutrition, serious infections requiring hospitalization in the month prior, serious chronic illness, or a personal or family history of allergy to cow’s milk or infant formula, eczema, allergic rhinitis or asthma.
We conducted a census in the District of Independencia to determine which households included a child ≤18 months old. Then, nurses conducted a food-intake survey to determine which children were weaned. Eligible families were visited by a study nurse who explained the protocol, answered questions, and obtained written informed consent from both parents.
Immediately after recruitment patients were assigned a study number that had been previously randomly assigned to bLF or placebo with fixed, equal allocation to each group and blocked randomization with block size of 4, prepared by a third party. Only the research pharmacist knew the randomization.
Community health workers visited each child 6 days/week (Monday through Saturday), twice daily (morning and afternoon) to give the coded preparations under supervision to ensure compliance. Children received 0.5g twice a day of bLF or placebo (diluted in 25 mL of water). The dose of lactoferrin was chosen based on the estimated amount consumed by a breastfeeding 12 month old.
The bLF preparation (Tatua Co-operative Dairy Co, Ltd, Morrinsville, New Zealand) is a freeze-dried protein purified directly from fresh bovine milk (iron saturation 10–20%). It is a salmon pink colored bland tasting powder produced under food grade conditions meeting ISO9001 standards. Maltodextrin (Montana S.A., Lima, Peru), a carbohydrate made from corn starch, was used as placebo. Both bLF and maltodrextrin were mixed with sugar, a strawberry flavor and pink food coloring agent, to make the preparations appear and taste identical. Screw top opaque plastic containers with a one month supply were prepared by a food processing company under good manufacturing practices (Montana S.A., Lima, Peru). Children received their normal diet including cow´s milk; however, commercially available cow´s milk does not provide a significant additional dose of bLF.
The physicians, nurses, community health workers, parents, and laboratory personnel were blinded to treatment assignment of each child throughout the study period. The data manager, statistician, and all investigators remained blinded to group assignment until the end of data analysis.
Diarrhea was defined as presence of ≥3 loose or watery stools in 24-hrs or ≥1 loose stool containing blood. An episode was considered to have started when a diarrhea day was preceded by at least 2 consecutive days without diarrhea and ended when the child had 3 consecutive days without any loose stool. All days between the start and ending day were considered part of the episode even if there was no diarrhea on a given day. Persistent diarrhea was defined as lasting for ≥14 days. Severe diarrhea was defined by the presence of ≥6 loose or watery stools in 24-hrs with vomiting, grossly bloody stools, documented fever (>39°C), or hospitalization for de hydration. Dehydration was assessed using WHO guidelines based on skin turgor, mental status and thirst, and was categorized as none, mild, moderate or severe. We used a Modified Ruuska-Vesikari score (MRV)22 to deter mine severity. For assessment of bLF effect on growth, z-scores of height-for-age (HFA) and weight-for-height (WFH) were used, based on WHO 2006 growth standards.
The primary study outcome was diarrhea incidence during the 6-month intervention. The secondary outcome was HFA and WFH z-scores. Additional diarrhea outcomes were longitudinal prevalence, duration, severity, dehydration, and prevalence of loose stools.
The community health workers performed daily home visits to record data on diarrhea, hydration, and sign/symptoms suggesting possible allergy to study interventions. Community health workers received training in basic health issues in order to give health education. The community health workers and parents were instructed to bring the child to the emergency room or study clinic if severe diarrhea developed. During episodes a stool sample was collected and the child was treated with ORS and/or antimicrobials as clinically indicated. Zinc therapy is not routinely used in Peru. Monthly stool samples were collected in the absence of gastrointestinal symptoms (± 7days) to evaluate colonization.
Stools were analyzed at the Enteric and Nutrition Laboratory - Tropical Medicine Institute “Alexander von Humboldt” in Lima, for common enteropathogens using conventional microbiological procedures. Rotavirus and adenovirus were determined using immunochromatography (Operon, Huerva-Zaragoza, Spain). Norovirus was detected by PCR using previously described primers.23 Diarrheagenic E. coli were diagnosed using a multiplex real time PCR.24 Parasites were determined by direct microscopy, stains for Coccidia and concentration methods for Strongyloides. Children were evaluated monthly by a pediatrician at the Outpatient Clinic for a history, exam and growth measurements. Children were weighed nude on the same calibrated infant scale (to the nearest 0.01 Kg), and length measured supine using standard length-height measuring boards. Personnel who performed the growth measurements were standardized twice yearly.
Poisson regression was used to model the relationship between expected number of diarrhea episodes of treatment versus placebo group. For sample size calculation we used a formula developed by Signorini25, based on one-sided hypothesis, which is consistent with our study. For sample size estimates we had assumed that there would be 3 diarrhea episodes/child/year in the placebo group, so that the number of children needed for a one sided test with a type I error (α) of 0.05 and a power (1-β) of 0.80 to detect a 25% reduction in the diarrhea episodes was 211 children in each group. We had projected that there would be a 30% dropout rate; we therefore planned to recruit 301 children/group. Partial information from dropouts has been used in the Poisson regression and in all analyses.
Analyses
Data were entered into an MSSQL database and were reviewed using SQL and VBS consistency checking programs. Patient, visit, result and episode analytical files were extracted to SPSS SAV binary format. Descriptive data tabulation comparing baseline and outcome variables between groups was made using SPSS V15.0. Statistical testing was made using R 2.13.1. Fisher Exact test for binary outcomes, Poisson test for rate outcomes, Student t-test for continuous outcomes (log-transformed if significant to Levene test) were used. To summarize the comparisons, estimates of ratios or differences for proportions, rates or means with their 95% confidence limits were made. Extended models for testing multivariate hypothesis are described in the Results. The Data Safety Monitoring Board (DSMB) met every 6 months to review data for safety and study compliance. Children experiencing a severe adverse event were referred to the DSMB for their judgment about continuation of the study.
The study was approved by Institutional Review Boards of the University of Texas Health Science Center in Houston and Universidad Peruana Cayetano Heredia in Lima; by the Direccion de Salud Lima Ciudad; Instituto Nacional de Salud-Peru ; and Direccion General de Medicamentos, Insumos y Drogas-Peru; and was registered at Clinicaltrials.gov (NCT00560222).
RESULTS
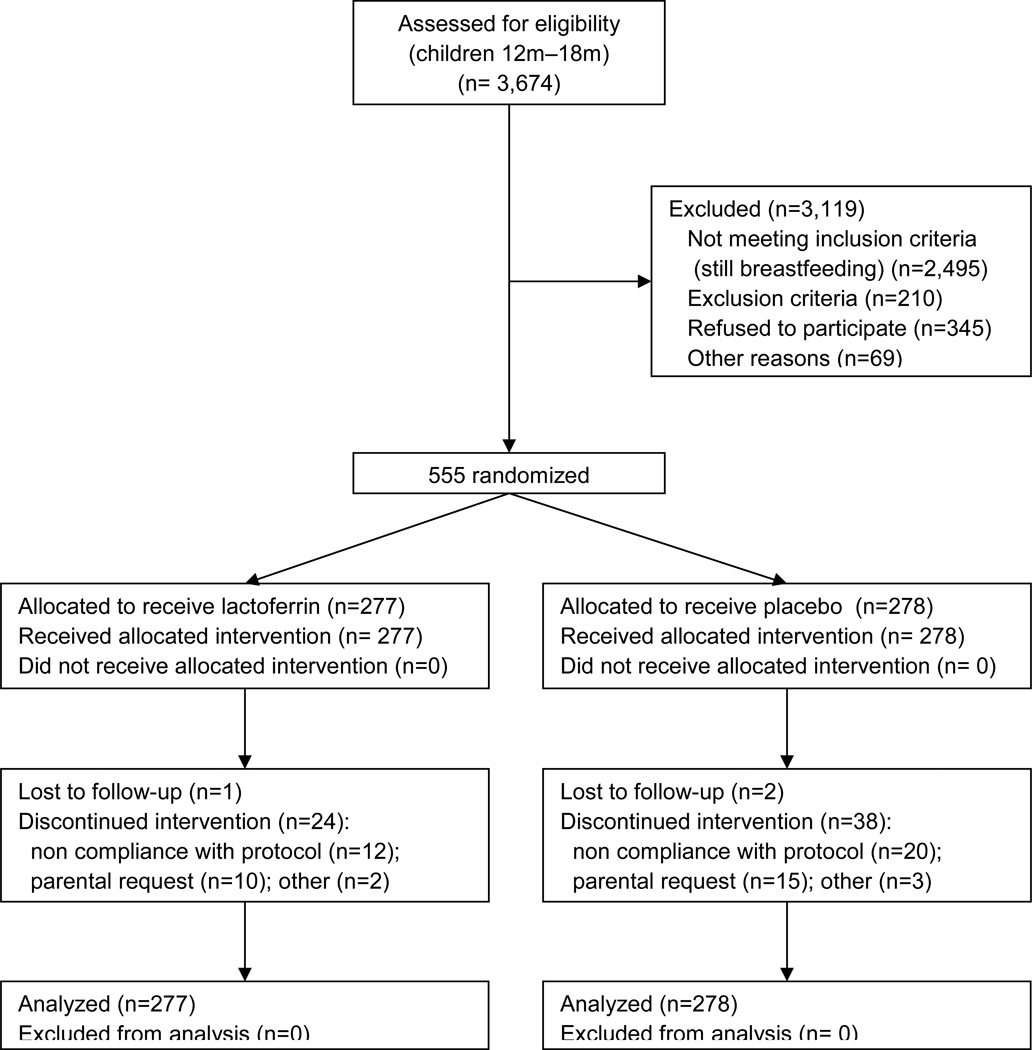
The study was conducted from January 2008 through May 2011. The census of 52,144 households found 3,674 children in the targeted age range. The food-intake survey found 2,495 children still breastfeeding (67.9%) leaving 1,179 eligible children (Figure). A lower than expected enrollment rate together with a much lower than expected drop out rate and much higher than expected illness rate, resulted in 555 rather than 602 children enrolled; 277 were randomized to bLF and 278 to placebo. Eighty nine baseline demographic and socio-economic characteristics and risk factors for diarrhea were compared by Kruskal-Wallis test; 8 had p<0.05, only WFH and diet intake of other micronutrients had p<0.01 (Table I). There were no reported reasons to suspect any loss of comparability introduced during the study. A set of 56 baseline variables (including socioeconomic, infant feeding, diarrhea history) was pooled using principal components analysis into two composite factors which were compared between groups using Wilcoxon's test, finding no significant difference (p 0.65 and 0.96).
Figure 1.
Study flow diagram. Enrollment: 3,674 children were assessed for eligibility. Allocation: 277 were randomized to lactoferrin and 278 to placebo; all received the allocated intervention. Follow-up: there were 25 and 40 drop-outs in the lactoferrin and placebo groups respectively. None were excluded from the analysis.
Table 1.
Baseline demographic and socio-economic characteristics, and risk factors for diarrhea in bLF and placebo groups.
| bLF | Placebo | ||
|---|---|---|---|
| Age at enrollment | Mean ± SD | 15.76 ± 2.08 | 16.07 ± 2.09 |
| Sex | Female, n (%) | 137 (49.5%) | 121 (43.5%) |
| Baseline anthropometry | Weight in Kg, mean ± SD | 10.10 ± 1.21 | 10.43 ± 1.23 |
| Height in cm, mean ± SD | 77.48 ± 3.22 | 78.03 ± 3.19 | |
| Head circumference in cm, mean ± SD | 45.98 ± 1.41 | 46.54 ± 1.46 | |
| Height-for-age (HFA) z score, mean ± SD | −0.60 ± 0.92 | −0.56 ± 0.99 | |
| Weight-for-height (WFH) z score, mean ± SD | 0.29 ± 0.93 | 0.51 ± 0.92 | |
| Weight-for-age (WFA) z score, mean ± SD | −0.06 ± 0.91 | 0.12 ± 0.95 | |
| Mass body index (MBI) in Kg/m2, mean ± SD | 16.79 ± 1.35 | 17.09 ±1.34 | |
| Mass body index (MBI) z score, mean ± SD | 0.40 ± 0.93 | 0.63 ± 0.89 | |
| Breastfeeding | Breastfed prior to entry in study, n (%) | 265 (96.4%) | 266 (96.7%) |
| Duration of exclusive breastfeeding in months, mean ± SD | 4.74 ± 3.89 | 4.67 ± 3.85 | |
| Weaning age in months, mean ± SD | 10.18 ± 4.52 | 10.70 ± 4.59 | |
| Diarrhea history | N° of diarrhea episodes in the pre vious 6m, mean ± SD | 2.01 ± 3.95 | 1.87 ± 2.14 |
| N° of prior persistent diarrhea episodes, mean ± S D | 0.07 ± 0.32 | 0.12 ± 0.41 | |
| Household | N° of family members who live in the hous e, mean ± SD | 6.09 ± 2.74 | 6.24 ± 2.96 |
| N° of children < 5y who live in the house, mean ± SD | 1.59 ± 0.92 | 1.61 ± 0.81 | |
| Family monthly income in US $, mean ± SD | 242.02 ± 118.26 | 240.71 ± 127.86 | |
| Number of bedrooms, mean ± SD | 2.35 ± 1.57 | 2.27 ± 1.48 | |
| Pipe water supply inside the house, n (%) | 227 (83.5%) | 237 (86.2%) | |
| Sewer line inside the house, n (%) | 233 (85.7%) | 240 (87.3%) | |
| Electricity in the home, n (%) | 260 (95.6%) | 262 (95.3%) | |
| Refrigerator in home, n (%) | 158 (58.1%) | 145 (52.7%) | |
| Television in home, n (%) | 260 (95.6%) | 256 (93.1%) | |
| Cell phone, n (%) | 202 (74.3%) | 190 (69.1%) | |
| Chickens living inside the house, n (%) | 78 (28.8%) | 59 (21.5%) | |
| Caregiver | Mother is the primary caregiver, n (%) | 187 (67.8%) | 205 (74.3%) |
| Grandmother is the primary caregiver, n (%) | 44 (15.9%) | 45 (16.3%) | |
| Mother age in years, mean ± SD | 28.18 ± 6.48 | 28.64 ± 6.57 | |
| Mother works inside or outside the house, n (%) | 107 (38.9%) | 119 (43.5%) | |
| Daycare attendance, n (%) | 7 (2.6%) | 2 (0.7%) | |
| Parents education | Mother did not complete high school, n (%) | 60 (22.0%) | 67 (24.4%) |
| Mother completed high school, n (%) | 134 (49.1%) | 120 (43.6%) | |
| Father did not complete high school, n (%) | 61 (23.4%) | 54 (21.3%) | |
| Father completed high school, n (%) | 134 (51.3%) | 131 (51.8%) |
There were 91,446 child/days of observation: 46,545 bLF and 44,901 placebo. There were 65 drop outs (11.7%): 25 bLF (9.0%, 95% CI [5.9–13]) and 40 placebo (14.4%, 95% CI [10.5–19.1]), p=0.064 (Figure). The study compliance was: 98% for planned home visits, 90% for planned monthly clinic visits, and 92% for planned doses administered (Table II).
Table 2.
Follow- up of patients: study compliance, clinical characteristics of the diarrheal episodes and growth outcomes.
| bLF | Placebo | |||
|---|---|---|---|---|
| Follow-up and compliance | ||||
| Home visits | Total days of observation | 46,545 | 44,901 | |
| Number of daily home visits | 37,709 | 36,401 | ||
| Daily home visits, mean ± SD per child | 168.03 ± 40.55 | 161.51 ± 48.77 | ||
| Compliance (actual /planned visits), mean ± SD | 0.98 ± 0.10 | 0.98 ± 0.08 | ||
| Medical visits | Number of planned monthly clinic visits | 1,756 | 1,707 | |
| Planned monthly clinic visits, mean ± SD per child | 6.34 ± 1.50 | 6.14 ± 1.73 | ||
| Number of clinic visits for illness | 734 | 724 | ||
| Sick visits, mean ± SD per child | 2.65 ± 2.47 | 2.60 ± 2.36 | ||
| Compliance (actual/ planned monthly clinic visits), mean ± SD | 0.91 ± 0.21 | 0.88 ± 0.25 | ||
| Treatment | Total number of doses received | 75,320 | 72,574 | |
| Number of doses received, mean ± SD per child | 271.91 ± 69.51 | 261.06 ± 81.57 | ||
| Compliance (doses received/planned), mean ± SD | 0.94 ± 0.24 | 0.91 ± 0.28 | ||
| Clinical characteristics of the diarrheal episodes | ||||
| Number of episodes | 646 | 589 | ||
| Duration in days | Median (minimum - maximum) | 4 (1 – 28) | 4 (1 – 62) | |
| 1 – 3 days, n (%) | 320 (49.5%) | 266 (45.2%) | ||
| 4 – 6 days, n (%) | 174 (26.9%) | 171 (29.0%) | ||
| 7 – 13 days, n (%) | 120 (18.6%) | 116 (19.7%) | ||
| 14 – 20 days, n (%) | 27 (4.2%) | 22 (3.7%) | ||
| ≥ 21 days, n (%) | 5 (0.8%) | 14 (2.4%) | ||
| Loose stools | Mean ± SD per episode | 13.3 ± 13.0 | 14.7 ± 15.0 | |
| Mean ± SD per day during episode | 3.0 ± 1.3 | 3.1 ± 1.5 | ||
| Maximum number per day, mean ± SD | 4.6 ± 2.3 | 4.7 ± 2.3 | ||
| Vomiting | Mean ± SD per episode | 0.6 ± 1.9 | 0.5 ± 1.7 | |
| Median per episode (minimum - maximum) | 0 (0 – 21) | 0 (0 – 24) | ||
| Mean ± SD per day during episode | 0.2 ± 0.5 | 0.1 ± 0.7 | ||
| Blood in feces | Episodes with bloody stools, n (%) | 27 (4.2%) | 18 (3.1%) | |
| Fever | Episodes with fever, n (%) | 99 (15.3%) | 89 (15.1%) | |
| Dehydration | Moderate or severe (WHO), n (%) | 6 (1.0%) | 15 (2.6%) | |
| Severity score | Moderate (MRV), n (%) | 72 (11.1%) | 71 (12.1%) | |
| Severe (MRV), n (%) | 4 (0.6%) | 5 (0.8%) | ||
| Severe episode, by study definition, n (%) | 208 (32.2%) | 200 (34.0%) | ||
| Growth outcomes: WHO 2006 z scores | ||||
| HFA z | Initial | −0.60 ± 0.92 | −0.56 ± 0.99 | |
| 1 month | −0.62 ± 0.92 | −0.59 ± 0.95 | ||
| 2 months | −0.67 ± 0.94 | −0.58 ± 0.98 | ||
| 3 months | −0.66 ± 0.91 | −0.56 ± 0.96 | ||
| 4 months | −0.64 ± 0.89 | −0.53 ± 0.93 | ||
| 5 months | −0.65 ± 0.89 | −0.54 ± 0.96 | ||
| 6 months | −0.57 ± 0.88 | −0.48 ± 0.95 | ||
| WFA z | Initial | 0.29 ± 0.93 | 0.51 ± 0.92 | |
| 1 month | 0.28 ± 0.93 | 0.52 ± 0.89 | ||
| 2 months | 0.26 ± 0.87 | 0.51 ± 0.85 | ||
| 3 months | 0.28 ± 0.91 | 0.61 ± 0.86 | ||
| 4 months | 0.28 ± 0.88 | 0.61 ± 0.86 | ||
| 5 months | 0.29 ± 0.87 | 0.60 ± 0.86 | ||
| 6 months | 0.25 ± 0.90 | 0.56 ± 0.89 | ||
MRV, Modified Ruuska-Vesikari score; HFAz, height-for-age z score (mean ± SD); WFHz, weight-for-height z score (mean ± SD)
1,235 diarrhea episodes occurred (646 bLF and 589 placebo), with an average duration of 5.04 ±4.79 days; 47.4% of episodes lasted ≤3 days and 5.5% were persistent; 33% of episodes were severe based on our study definition (Table II). There was no difference in diarrhea incidence between groups; 5.4 vs. 5.2 episodes/child/year for bLF and placebo, respectively (p=0.375). However, there were small but significant differences in duration, longitudinal prevalence, dehydration and prevalence of loose stool, with less overall diarrhea burden with bLF (Table III). This decrease in diarrhea severity was not associated with a decrease in ORS and/or antimicrobial usage.
Table 3.
Comparison of diarrhea outcomes between the bLF and placebo groups.
| bLF (95% CI) |
Placebo (95% CI) |
Type* | Comparison (95% CI) |
P | |
|---|---|---|---|---|---|
| Incidence, episodes/child/year | 5.43 (5.02 – 5.86) | 5.15 (4.74 – 5.59) | R | 1.05 (0.94 – 1.18) | 0.375 |
| Duration, days | 4.76 (4.41 – 5.15) | 5.34 (4.93 – 5.80) | I | 0.89 (0.80 – 0.99) | 0.046 |
| Average prevalence, % of days with diarrhea | 6.6 (6.4 – 6.8) | 7.0 (6.8 – 7.2) | P | −0.4 (−0.7 – −0.1) | 0.017 |
| Episodes with moderate or severe dehydration, % | 1.0 (0.4 – 2.1) | 2.6 (1.5 – 4.3) | P | −1.6 (−3.3 – −0) | 0.045 |
| Episodes with severe diarrhea, % | 32.2 (28.6 – 36.0) | 34.0 (30.1 – 37.9) | P | −1.8 (−7.2 – 3.7) | 0.545 |
| Episode severity score (MVS), mean | 4.89 (4.67 – 5.11) | 5.05 (4.83 – 5.28) | M | −0.16 (−0.48 – 0.16) | 0.625 |
| Bloody diarrhea, days with blood/child/year | 0.28 (0.19 – 0.38) | 0.30 (0.21 – 0.42) | U | 0.91 (0.56 – 1.49) | 0.787 |
| Total loose stools, loose stools /child/year | 95.0 (91.3 – 95.0) | 98.6 (95.0 – 98.6) | U | 0.95 (0.93 – 0.98) | <0.001 |
R: estimation by incidence rate, comparison by bLF/placebo (P) rate ratio, p from Poisson test; I: estimation by duration as inverse of recovery rate, comparison by bLF/P rate ratio, p from Poisson test; P: estimation by binomial proportion, comparison by bLF-P difference of proportions, p from Fisher test; M: estimation by arithmetic mean, comparison by bLF-P difference of means, p unequal v Student test; U: estimation by rate, comparison by bLF/P rate ratio, p from Poisson test
We studied 915 diarrhea stool samples (74% of episodes had a sample collected). (Table IV). There were no differences in incidence, prevalence or clinical characteristics for any pathogen related to group assignment. There were no differences in prevalence of colonizing pathogens between diarrhea and control groups based on 2,734 stool samples collected in the absence of diarrhea (Table IV).
Table 4.
Pathogens isolated from diarrheal and colonization samples
| Pathogen | DIARRHEA | COLONIZATION (healthy controls) | |||||
|---|---|---|---|---|---|---|---|
| bLF N = 484 n (%) |
Placebo N = 431 n (%) |
Total N = 915 n (%) |
bLF N = 1,396 n (%) |
Placebo N = 1,338 n (%) |
Total N = 2,734 n (%) |
||
| Bacteria | Campylobacter | 61 (12.6) | 36 (8.4) | 97 (10.6) | 107 (7.7) | 109 (8.1) | 216 (7.9) |
| Shigella | 24 (5.0) | 36 (8.4) | 60 (6.6) | 12 (0.9) | 18 (1.3%) | 30 (1.1) | |
| Vibrio | 2 (0.4) | 0 (0) | 2 (0.2) | 3 (0.2) | 3 (0.2) | 6 (0.2) | |
| Other bacteria† | 4 (0.8) | 6 (1.4) | 10 (1.1) | 16 (1.1) | 22 (1.6) | 38 (1.4) | |
| EPEC | 57 (11.8) | 47 (11.0) | 104 (11.4) | 138 (10.0) | 169 (12.7) | 307 (11.3) | |
| EAEC | 42 (8.7) | 34 (7.9) | 76 (8.4) | 128 (9.2) | 124 (9.3) | 252 (9.3) | |
| ETEC | 37 (7.7) | 26 (6.1) | 63 (6.9) | 55 (4.0) | 59 (4.4) | 114 (4.2) | |
| DAEC | 14 (2.9) | 13 (3.0) | 27 (3.0) | 28 (2.0) | 21 (1.6) | 49 (1.8) | |
| EIEC | 5 (1.0) | 2 (0.5) | 7 (0.8) | 8 (0.6) | 12 (0.9) | 20 (0.7) | |
| STEC | 3 (0.6) | 2 (0.5) | 5 (0.5) | 14 (1.0) | 20 (1.5) | 34 (1.3) | |
| Virus | Rotavirus | 11 (3.2) | 15 (4.7) | 26 (3.9) | ND | ND | ND |
| Adenovirus | 23 (3.6) | 23 (3.8) | 46 (3.7) | ND | ND | ND | |
| Norovirus* | 141 (34.3) | 134 (35.5) | 275 (35.0) | ND | ND | ND | |
| Parasites | Giardia | 38 (7.9) | 20 (4.6) | 58 (6.3) | 114 (8.2) | 89 (6.7) | 203 (7.4) |
| Blastocystis | 8 (1.7) | 9 (2.2) | 17 (1.9) | 25 (1.8) | 34 (2.5) | 59 (2.2) | |
| Cryptosporidium | 4 (0.9) | 1 (0.2) | 5 (0.6) | 5 (0.4) | 5 (0.4) | 10 (0.4) | |
| Cyclospora | 1 (0.2) | 1 (0.2) | 2 (0.2) | 2 (0.1) | 0 (0) | 2 (0.1) | |
| Strongyloides | 1 (0.2) | 0 (0) | 1 (0.1) | 1 (0.1) | 0 (0) | 1 (0.0) | |
| Other parasites‡ | 18 (3.9) | 11 (2.6) | 29 (3.3) | 55 (3.9) | 69 (5.2) | 124 (4.5) | |
ND, no data (viruses were not studied in the healthy controls samples).
Norovirus: 17.1% G-I, 82.9% G-II.
Other bacteria: Aeromonas, Plesiomonas, Salmonella.
Other parasites: Chilomastix,, Endolimax, Entamoeba, Enterobius, Diphylobothrium, Hymenolepis, Trichuris, Isospora.
Anthropometric z-scores (Table II) were tested in a linear mixed model regression having intercept, treatment group, time since start of supplementation and the product of both as fixed effect terms plus individual child intercept as random effect term. For HFA, significant differences (p=0.010) by group slope, but not intercept (p=0.525), were found. For WFH, significant differences by group intercept (p=0.002), but not slope (p=0.050), were found. Additional adjustment by adding baseline anthropometry, age upon admission, day of year and completion status confirmed the treatment group slope significance in HFA (p<0.001) and dismissed any treatment significance in WFH. Modeling indicates that the bLF group had a slightly lower (0.12z, 95%CI [0.07–0.17]) HFA than the placebo group at the end of treatment.
During planed monthly outpatient clinic visits and sick visits there were no differences in the prevalence of common pediatric diagnosis between the bLF and placebo groups. Occurrence of possible allergic reactions, prevalence of skin allergy or eczema, allergic rhinitis, and bronchospasm were similar between groups as was use of antihistamines and anti-asthma medications. There were 17 severe adverse events (SAE), 9 bLF and 8 placebo. All SAE were hospitalizations for common pediatric illnesses; none were considered related to the intervention by the DSMB.
DISCUSSION
This study failed to achieve its primary objective of demonstrating decreased incidence of diarrheal disease with bLF as well as the secondary objective of demonstrating improved growth. However, measures of severity were positively affected although the benefit was small. The data suggest that chronic use of bLF such as is currently done in some infant formulas is unlikely to have a major impact on diarrhea in children. Lactoferrin without other breast milk factors may have limited value. Lactoferrin might have important benefits on immune or other functions, but its failure to improve growth does not support the concept that it is a major factor that could improve child health in this age group. Although adjusted analysis finds a clinically small difference in HFA, the authors do not unanimously agree on the interpretability, given the baseline WFH difference.
The small benefits noted in disease severity suggest that further studies ought to focus on lactoferrin as an adjunct to other measures aimed at management of acute or persistent diarrheal disease. A previous pediatric study of acute watery diarrhea showed that adding lysozyme and recombinant hLF expressed in rice to oral rehydration solution reduced the duration and recurrence of diarrhea.26 Our findings are in concordance with previous smaller, less intensively monitored trials. A 12wk study of 298 Japanese children showed no difference in incidence of rotaviral gastroenteritis, but duration of episodes and frequency and duration of vomiting were decreased with bLF.27 A study in 52 US infants receiving a bLF-enhanced formula for 12m found no differences in diarrhea incidence; however, there were significantly fewer lower respiratory tract illnesses in the bLF-fed compared with regular formula-fed infants.28 We had conducted a prior pilot trial of bLF for 9m in 52 Peruvian children. The bLF group had less Giardia burden and better HFA z-score.29 The current trial failed to confirm these preliminary findings.
The trial has some limitations. First, only one dose (1,000 mg/day), equivalent to the amount of LF in 100 mL of colostrum (10 mg/ml) or one liter of post colostral breast milk (1 mg/ml), was tested. This dose was chosen based on a preliminary pilot study of bLF for prevention of diarrhea.29 However, LF dosing has been very variable in previous pediatric clinical trials. Other studies have used as low as 10mg/100mL of milk, 100 mg/day, or as high as 1,000 mg every 8 hours.19 Therefore, although this dose seemed reasonable, it was potentially to low, especially for this age group. Obviously future studies should evaluate larger doses. Second, the intense observation, regular physician evaluations, and home health educational intervention may have modified risks so that the observed rates of diarrhea and growth may have been better than would have occurred in the absence of the trial. Third, the age range studied was narrow; bLF might have greater benefit in some other age range, such as neonates, because recent data has demonstrated an important effect of bLF on prevention of sepsis in preterm neonates.18 Children in the second year of life may digest bLF so that it has less impact on enteric pathogens; however, the processing of LF in the gut is not fully defined. Fourth, enteropathogens in Peru are similar to those in most of the world but different from the organism in Japan, North America, and Europe. In such settings it is possible that bLF could have a role in immunologically naïve populations. The high frequency of exposure to enteropathogens in Peru22 may have boosted pathogen specific immunity such that the effect of lactoferrin was lessened. A population with a lower frequency of infection might show a greater impact from bLF. Fifth, the use of bLF rather than hLF could be debated but we believe it is reasonable given that both have similar effects on diarrheal pathogens in multiple laboratory models.30 HLF and bLF are well characterized. They have 691 and 689 amino acids, respectively; the sequence identity is 69%; and the 3-D structures are very similar.31 Although differences in structural and biochemical properties exist, their bioactivity, as assessed in vitro or in animal models, is quite comparable.15,16 BLF and hLF have been evaluated in several clinical trials in children with various objectives: iron metabolism, anemia, fecal flora, enteric infections, immunomodulation in HIV children and neonatal sepsis.19 Although the efficacies have been variable in each trial (due to different study outcomes), there are no data on the effectiveness of bLF vs. hLF for the same outcome measured in a pediatric clinical trial. However, based on the in vitro and animal studies and the available data in humans, both LF preparations appear to be comparable on their likely effects on enteric infections.
In summary, although this study makes it unlikely that bLF can have a major role in prevention of diarrhea in children in the second year of life, it leaves open the possibility that lactoferrin could have a role in younger infants or as an adjunct to other measures in treatment of diarrheal episodes, especially for the treatment of prolonged and persistent diarrhea, which are associated with malnutrition and impaired neurodevelopment.
Acknowledgments
We would like to thank Dr. Edith Caballero, Head of the Centro de Salud Ermitaño Alto in Lima, and all the health care personnel at the Center for their support and collaboration in the study. We also thank the study pediatricians: Drs. Roger Hernandez, Alejandro Alvarez, Isolda Gonzales, Patricia Reyes, Karina Altamirano and Sandra Aedo; our Research Nurses Jenny Leon, Giuliana Ponte, Merly Aponte, Rocio Lucas and Gaby Cuicapusa, as well as the nurse aids and community health workers for their dedication and careful work in this project. We thank NAMRU-6 in Lima for the analysis of the norovirus samples. We also thank the members of the DSMB for their independent and critical review of the data and safety of the study.
Funded by National Institute of Child Health and Human Development, USA (Public Health Service award R01-HD051716). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHHD or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.Guerrant RL, Oriá RB, Moore SR, Oriá MO, Lima AA. Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev. 2008;66:487–505. doi: 10.1111/j.1753-4887.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS Bellagio Child Survival Study Group. How many child deaths can we prevent this year? Lancet. 2003;362:65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- 4.Victora CG, Smith PG, Vaughan JP, Nobre LC, Lombardi C, Teixeira AM, et al. Infant feeding and deaths due to diarrhea. A case-control study. Am J Epidemiol. 1989;129:1032–1041. doi: 10.1093/oxfordjournals.aje.a115207. [DOI] [PubMed] [Google Scholar]
- 5.Morrow AL, Rangel JM. Human milk protection against infectious diarrhea: implications for prevention and clinical care. Semin Pediatr Infect Dis. 2004;15:221–228. doi: 10.1053/j.spid.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Lönnerdal B. Bioactive proteins in human milk: mechanisms of action. J Pediatr. 2010;156:26–30. doi: 10.1016/j.jpeds.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Brock JH. The physiology of lactoferrin. Biochem Cell Biol. 2002;80:1–6. doi: 10.1139/o01-212. [DOI] [PubMed] [Google Scholar]
- 8.Lönnerdal B. Nutritional roles of lactoferrin. Curr Opin Clin Nutr Metab Care. 2009;12:293–297. doi: 10.1097/MCO.0b013e328328d13e. [DOI] [PubMed] [Google Scholar]
- 9.Berlutti F, Pantanella F, Natalizi T, Frioni A, Paesano R, Polimeni A, et al. Antiviral properties of lactoferrin--a natural immunity molecule. Molecules. 2011;16:6992–7018. doi: 10.3390/molecules16086992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appelmelk BJ, An YQ, Geerts M, Thijs BG, de Boer HA, MacLaren DM, et al. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994;62:2628–2632. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandenburg K, Jürgens G, Müller M, Fukuoka S, Koch MH. Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin. Biol Chem. 2001;382:1215–1225. doi: 10.1515/BC.2001.152. [DOI] [PubMed] [Google Scholar]
- 12.Gomez HF, Ochoa TJ, Carlin LG, Cleary TG. Human lactoferrin impairs virulence of Shigella flexneri. J Infect Dis. 2003;187:87–95. doi: 10.1086/345875. [DOI] [PubMed] [Google Scholar]
- 13.Ochoa TJ, Noguera-Obenza M, Ebel F, Guzman CA, Gomez HF, Cleary TG. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect Immun. 2003;71:5149–5155. doi: 10.1128/IAI.71.9.5149-5155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochoa TJ, Brown EL, Guion CE, Chen JZ, McMahon RJ, Cleary TG. Effect of lactoferrin on enteroaggregative E. coli (EAEC) Biochem Cell Biol. 2006;84:369–376. doi: 10.1139/o06-053. [DOI] [PubMed] [Google Scholar]
- 15.Steijns JM, van Hooijdonk AC. Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br J Nutr. 2000;84:11–17. doi: 10.1017/s0007114500002191. [DOI] [PubMed] [Google Scholar]
- 16.Tomita M, Wakabayashi H, Yamauchi K, Teraguchi S, Hayasawa H. Bovine lactoferrin and lactoferricin derived from milk: production and applications. Biochem Cell Biol. 2002;80:109–112. doi: 10.1139/o01-230. [DOI] [PubMed] [Google Scholar]
- 17.Hernell O, Lönnerdal B. Iron status of infants fed low-iron formula: no effect of added bovine lactoferrin or nucleotides. Am J Clin Nutr. 2002;76:858–864. doi: 10.1093/ajcn/76.4.858. [DOI] [PubMed] [Google Scholar]
- 18.Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302:1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- 19.Ochoa TJ, Peso A, Cruz K, Chea-Woo E, Cleary TG. Clinical studies of lactoferrin in children. Biochem Cell Biol. 2012;90:457–467. doi: 10.1139/o11-087. [DOI] [PubMed] [Google Scholar]
- 20.Gomez HF, Ochoa TJ, Herrera-Insua I, Carlin LG, Cleary TG. Lactoferrin protects rabbits from Shigella flexneri-induced inflammatory enteritis. Infect Immun. 2002;70:7050–7053. doi: 10.1128/IAI.70.12.7050-7053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosquito S, Ochoa TJ, Cok J, Cleary TG. Effect of bovine lactoferrin in Salmonella ser. Typhimurium infection in mice. Biometals. 2010;23:515–521. doi: 10.1007/s10534-010-9325-1. [DOI] [PubMed] [Google Scholar]
- 22.Ochoa TJ, Ecker L, Barletta F, Mispireta ML, Gil AI, Contreras C, et al. Age-related susceptibility to infection with diarrheagenic Escherichia coli among infants from Periurban areas in Lima, Peru. Clin Infect Dis. 2009;49:1694–1702. doi: 10.1086/648069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill VR, Mull B, Jothikumar N, Ferdinand K, Vinjé J. Detection of GI and GII noroviruses in ground water using ultrafiltration and TaqMan realtime RT-PCR. Food and Environmental Virology. 2010;2:218–224. [Google Scholar]
- 24.Guion CE, Ochoa TJ, Walker CM, Barletta F, Cleary TG. Detection of diarrheagenic Escherichia coli by use of melting-curve analysis and real-time multiplex PCR. J Clin Microbiol. 2008;46:1752–1757. doi: 10.1128/JCM.02341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Signorini Sample size for Poisson regression. Biometrika. 1991;78:446–450. [Google Scholar]
- 26.Zavaleta N, Figueroa D, Rivera J, Sánchez J, Alfaro S, Lönnerdal B. Efficacy of rice-based oral rehydration solution containing recombinant human lactoferrin and lysozyme in Peruvian children with acute diarrhea. J Pediatr Gastroenterol Nutr. 2007;44:258–264. doi: 10.1097/MPG.0b013e31802c41b7. [DOI] [PubMed] [Google Scholar]
- 27.Egashira M, Takayanagi T, Moriuchi M, Moriuchi H. Does daily intake of bovine lactoferrin-containing products ameliorate rotaviral gastroenteritis? Acta Paediatr. 2007;96:1242–1244. doi: 10.1111/j.1651-2227.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 28.King JC, Jr, Cummings GE, Guo N, Trivedi L, Readmond BX, Keane V, et al. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J Pediatr Gastroenterol Nutr. 2007;44:245–251. doi: 10.1097/01.mpg.0000243435.54958.68. [DOI] [PubMed] [Google Scholar]
- 29.Ochoa TJ, Chea-Woo E, Campos M, Pecho I, Prada A, McMahon RJ, et al. Impact of lactoferrin supplementation on growth and prevalence of Giardia colonization in children. Clin Infect Dis. 2008;46:1881–1883. doi: 10.1086/588476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochoa TJ, Cleary TG. Effect of lactoferrin on enteric pathogens. Biochimie. 2009;91:30–34. doi: 10.1016/j.biochi.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierce A, Colavizza D, Benaissa M, Maes P, Tartar A, Montreuil J, et al. Molecular cloning and sequence analysis of bovine lactotransferrin. Eur J Biochem. 1991;196:177–184. doi: 10.1111/j.1432-1033.1991.tb15801.x. [DOI] [PubMed] [Google Scholar]