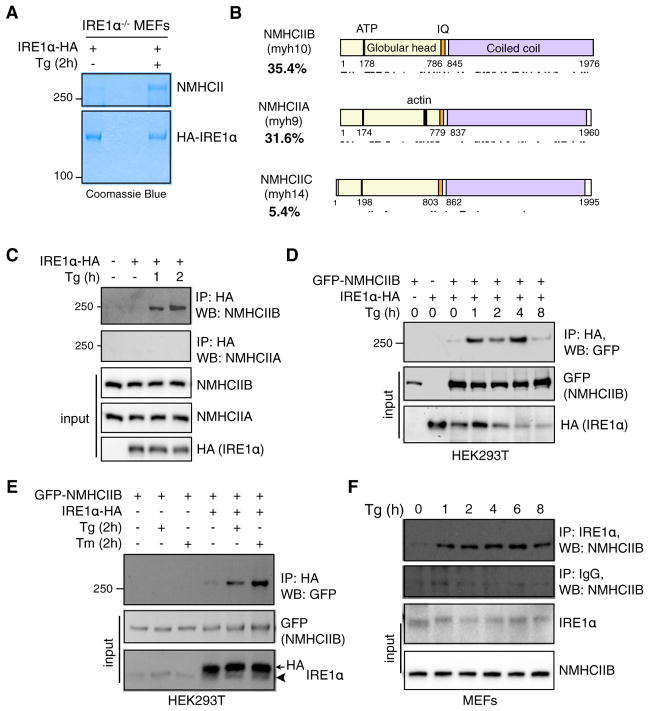
Figure 1. NMIIB Is an ER Stress-Induced IRE1α-Interacting Factor.
(A) Coomassie blue staining of immunoprecipitates (IPs) of IRE1α−/− MEFs stably expressing C-terminal hemagglutinin (HA)-tagged IRE1α untreated or treated with 300 nM Tg for 2 hr. Unknown Tg-specific band at 3250 kDa was excised and identified as NMHCII using tandem mass spectrometry (MS/MS) analysis.
(B) Schematic of the functional domains of the three mammalian isoforms of NMHCII with respective peptide coverage recovered (indicated by lines below) from MS/MS. Figure is drawn to scale using sequence annotation data from UniProt.
(C) Western blot showing recovery of endogenous NMHCIIB and NMHCIIA from immunoprecipitates of HA-tagged IRE1α prepared from transiently transfected HEK293T cells treated with 150 nM Tg for the indicated time.
(D and E) Western blot showing recovery of GFP-tagged NMHCIIB from IPs of HA-tagged IRE1α prepared from transfected HEK293T cells treated with 300 nM Tg for the indicated time (D) or 300 nM Tg or 5 μg/ml Tm for 2 hr (E). Arrowhead points to endogenous protein.
(F) Western blot showing recovery of endogenous NMHCIIB from IPs of endogenous IRE1α prepared from MEFs treated with 150 nM Tg for the indicated time.
For (C)–(F), similar results were observed in 2–3 independent experiments.