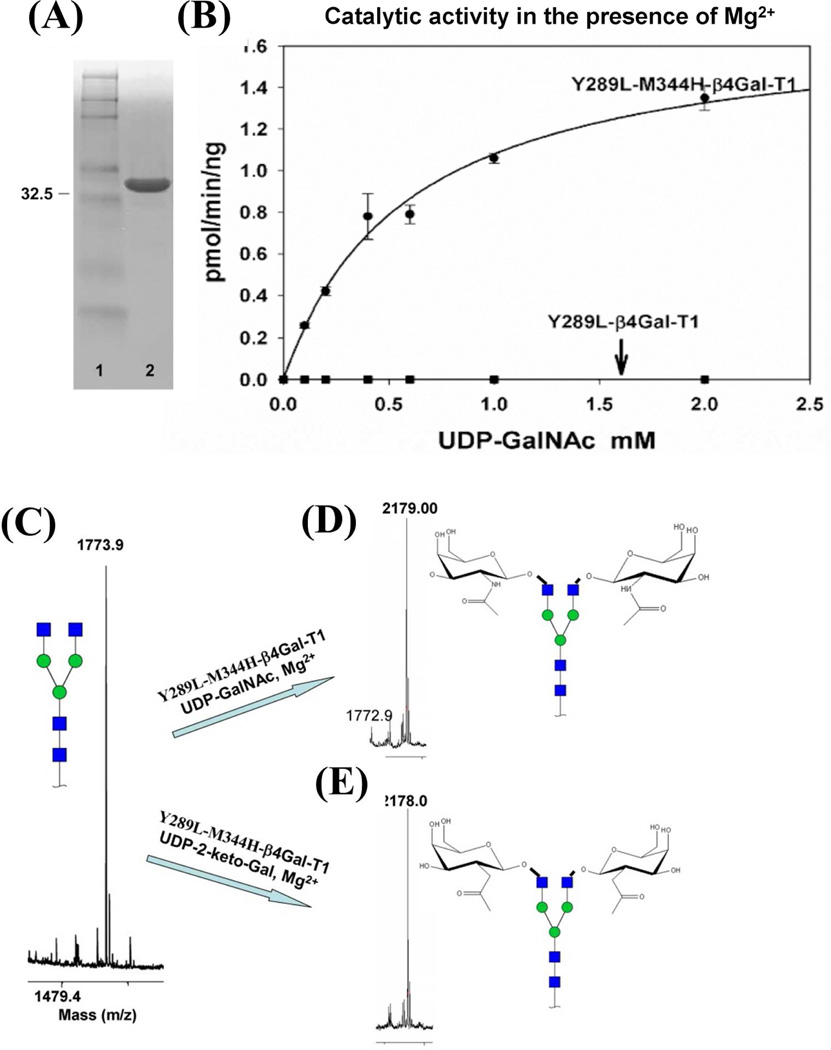
Figure 2.
The expression and the catalytic activity of the double mutant enzyme Y289L-M344H-β4GalT1. A: Nonreducing SDS-PAGE gel stained with Brilliant Blue G showing 10 µg of active in vitro refolded Y289L-M344-β4GalT1 mutant protein in lane 2, with the molecular weight marker in lane 1. B: Catalytic activity of the Y289L-β4Gal-T1 and Y289L-M344H-β4Gal-T1 proteins in the presence of MgCl2, UDP-GalNAc, and a 25 mM β-benzyl-GlcNAc as an acceptor substrate. The single mutant enzyme does not show any catalytic activity in the presence of Mg2+.MALDI-TOF spectra of the G0 form of the N-glycan acceptor substrate, C: and its G2 form of the product with GalNAc, D: and 2-keto-Gal sugars, E, in the catalytic reaction with the double mutant enzyme Y289L-M344-β4Gal-T1 in the presence of Mg2+. Major peaks are annotated with the carbohydrate structure that is shown in symbols for monosaccharides, according to the nomenclature adopted by the Consortium for Functional Glycomics.