Abstract
It is “normal” for old age to be associated with gradual decline in memory and brain mass. However, there are anecdotal reports of individuals who seem immune to age-related memory impairment, but these individuals have not been studied systematically. This study sought to establish that such cognitive SuperAgers exist and to determine if they were also resistant to age-related loss of cortical brain volume. SuperAgers were defined as individuals over age 80 with episodic memory performance at least as good as normative values for 50- to 65-year-olds. Cortical morphometry of the SuperAgers was compared to two cognitively normal cohorts: age-matched elderly and 50- to 65-year-olds. The SuperAgers’ cerebral cortex was significantly thicker than their healthy age-matched peers and displayed no atrophy compared to the 50- to 65-year-old healthy group. Unexpectedly, a region of left anterior cingulate cortex was significantly thicker in the SuperAgers than in both elderly and middle-aged controls. Our findings identify cognitive and neuroanatomical features of a cohort that appears to resist average age-related changes of memory capacity and cortical volume. A better understanding of the underlying factors promoting this potential trajectory of unusually successful aging may provide insight for preventing age-related cognitive impairments or the more severe changes associated with Alzheimer’s disease. (JINS, 2012, 18, 1081–1085)
Keywords: Structural magnetic resonance imaging (MRI), Neuropsychology, Dementia, Freesurfer, Elderly, Neuroimaging
INTRODUCTION
When used to describe older adults, the concept of “cognitively normal-for-age” suggests that decline is normal. That is, scores considered “normal” for an 80-year-old on many cognitive measures are markedly lower than normal scores for a 50-year-old, especially for tests of episodic memory (for a discussion on this concept, see Evans, Grodstein, Loewenstein, Kaye, & Weintraub, 2011). What is poorly understood is whether age-related decline is inevitable beyond age 80 or whether there is an alternative trajectory that resists the cognitive and anatomic changes characteristic of normal aging.
This question was addressed in a prospective study of individuals called SuperAgers, aged 80 or older, selected for scores on tests of episodic memory that were above-average-for-age, at a level considered at least normal for individuals 20–30 years younger, and whose scores in other cognitive domains were at least average-for-age. While several studies of successful aging exist (for relevant reviews, see Depp & Jeste, 2006; Kaup, Mirzakhanian, Jeste, & Eyler, 2011; Rowe & Kahn, 1997), few incorporate cognitive function into their definition and none require memory performance to be at least as good as those of individuals two to three decades younger.
This study examined whether the SuperAgers’ cortical morphometry was distinct from typical age-related atrophy by quantitatively comparing their structural magnetic resonance imaging (MRI) scans to those of two cognitively normal reference groups: elderly controls and middle-aged controls.
METHODS
Participants
Participants were identified based on chronologic age (Super-Agers: ≥80 years old, Middle-aged Controls: 50–65 years old, Elderly Controls: age-matched to SuperAger cohort), availability of a 3 Tesla (T) structural MRI scan and neuropsychological performance (described below). All participants were required to have preserved activities of daily living and lacked clinical or structural evidence of neurologic or psychiatric disease.
SuperAgers and middle-aged controls were community dwellers, recruited through Northwestern’s Alzheimer’s Disease Center (ADC) Clinical Core, community lectures, and word of mouth. Twelve SuperAgers and 14 middle-aged controls met our criteria and were included in the analysis.
Since Northwestern’s ADC does not routinely perform structural MRI scans on healthy elderly participants, data were obtained through the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. Cognitively normal and impaired individuals have been recruited into ADNI from over 50 sites across the United States and Canada, including Northwestern’s ADC, and their imaging and neuropsychological data are made available to approved investigators (http://adni.loni.ucla.edu/). Eleven individuals met criteria; one participant was subsequently excluded because their MRI data contained fatal defects.
Written informed consent was obtained for all participants, which was approved by the IRB of each participating center.
The neuropsychological measures were chosen on the basis of their relevance for cognitive aging and for early detection of Alzheimer’s disease (AD) (Weintraub, Wicklund, & Salmon, 2011). The delayed verbal recall score of the Rey Auditory Verbal Learning Test (RAVLT) was used to assess episodic memory. SuperAgers were required to perform at or above average normative values for individuals in their 50s and 60s (midpoint age = 61; RAVLT delayed-recall raw score ≥9) (Schmidt, 2004), while healthy middle-aged controls and elderly controls were required to score within one standard deviation of the average range for their age and education according to published normative values (Figure 1A; Schmidt, 2004). The 30-item Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 1983), Trail Making Test Part B (Randolph, 1998), and the Category Fluency Test (Morris et al., 1989) were used to assess cognitive function in non-memory domains. All participants were required to score within one standard deviation of the average range for their age and education on each of these measures according to published normative values (Heaton, Miller, Taylor, & Grant, 2004; Randolph, 1998; Saxton et al., 2000).
Fig. 1.
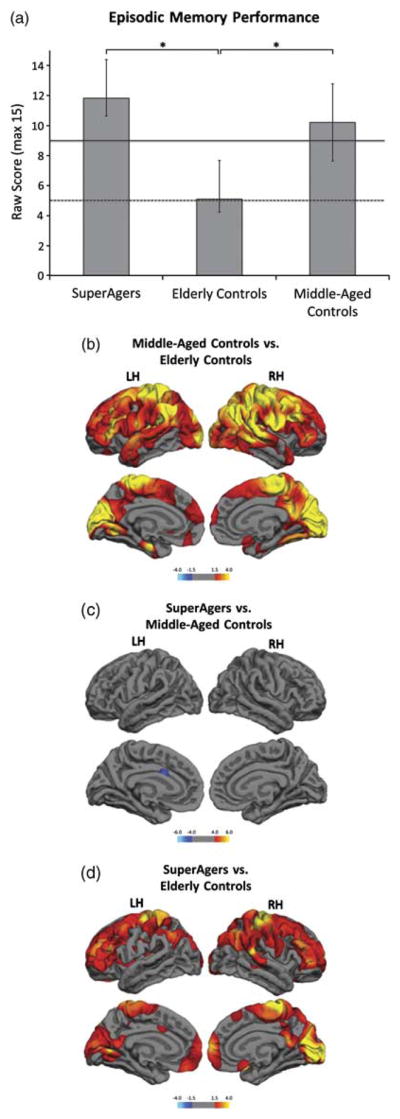
Episodic memory performance and differences in cortical thickness by group. (a) Group average delayed recall scores on the RAVLT word list show SuperAgers performing significantly better than elderly controls. There is no significant difference in episodic memory performance between SuperAgers and middle-aged controls. The solid and dotted lines represent the average normative values for a 60-year-old and an 80-year-old, respectively (Schmidt, 2004). * represents significant differences at p <.05. (b) Red and yellow represent significantly thinner cortex in elderly controls compared to middle-aged controls. (c) There is no significant thinning in the SuperAging group in either hemisphere compared to middle-aged controls. A region within the anterior cingulate (blue) is significantly thicker in SuperAgers when compared to middle-aged controls. (d) Red and yellow represent significantly thinner cortex in elderly controls compared to SuperAgers. False discovery rate (FDR) was set at 0.05 for each MRI analysis. Color bars display significance using as −log(10) p value.
MRI Acquisition
All subjects received T1-weighted three-dimensional MP-RAGE sequences (TR = 2300 ms, TE = 2.86 ms, flip angle = 9°, FoV = 256 mm) (Jack et al., 2008). Cortical thickness was calculated by measuring the distance between representations of the white-gray and pial-CSF boundaries across each point of the cortical surface using the image analysis suite FreeSurfer (version 4.5.0; http://surfer.nmr.mgh.harvard.edu/; Fischl & Dale, 2000). This sensitive and well-validated method uses both intensity and continuity information from the entire MR volume, and produces thickness maps capable of detecting submillimeter differences between groups (Kuperberg et al., 2003).
Statistical Analysis
Statistical surface maps were generated using a general linear model that displays differences in cortical thickness between two groups for each vertex along the surface representations. A false discovery rate of 0.05 was used to adjust for multiple comparisons (Genovese, Lazar, & Nichols, 2002).
Cortical volume, a summary measure of cortical integrity (Desikan et al., 2006), was calculated for each participant, normalized by intracranial volume (Buckner et al., 2004) and compared between groups using an analysis of variance (ANOVA). Pearson correlations were used to examine the relationship between cortical morphometry measures and memory performance.
All statistical analyses were performed within FreeSurfer or with PASW 19.0 (SPSS).
RESULTS
Twelve SuperAgers (average age = 83.5 ±3.0 years), 10 elderly controls (average age = 83.1 ±3.4 years), and 14 middle-aged controls (average age = 57.9 ±4.3 years) were included in the analyses. There were no significant differences in education among the groups (average years of education for Super-Agers = 14.8 ±2.4, elderly controls = 17.5 ±2.2, middle-aged controls = 16.1 ±2.9).
As defined by our experimental design, episodic memory performance for the elderly and middle-aged controls was within one standard deviation of published normative values (Schmidt, 2004), while SuperAgers’ performed at least as well as middle-aged controls (p = .09) and significantly better than elderly controls (p <.001; Figure 1A). All participants scored at least within one standard deviation of the average range for their age and education on non-memory testing, confirming the absence of cognitive impairment. One-way ANOVAs showed significant differences between the three groups on Trails B but not on Category Fluency or the BNT (Trails B: F = 4.1, p = .027, Average Score Super-Agers = 96.2 ±46.1, Middle Age Controls = 61.8 ±22.0, Elderly Controls = 128.5 ±92.7; Category Fluency: F = 3.0, p = .064, Average Score SuperAgers = 22.4 ±6.0, Middle Age Controls = 23.7 ±5.6, Elderly Controls = 18.4 ±3.8; BNT: F = 0.9, p = .424, Average Score SuperAgers = 28.7 ±1.1 Middle Age Controls = 28.9 ±1.0, Elderly Controls = 28.0 ±2.5). Post hoc tests revealed that the elderly controls were significantly slower than the middle-aged controls (p <.05) on Trails B but there were no other significant differences between the groups. Thus, as a group, the SuperAgers did not differ from the middle-aged controls on non-memory performance.
Middle-Aged Controls Versus Elderly Controls
The whole-brain cortical thickness comparison conducted between elderly controls and middle-aged controls revealed, as expected, significant atrophy in the older cohort in multiple regions across the frontal, parietal and occipital lobes, including medial temporal regions important for memory function (Figure 1B). This pattern of atrophy in the cognitively normal elderly controls is consistent with previous findings (e.g., Salat et al., 2004), and presumably reflects the anatomical substrate of the age-related decline of cognitive function, including memory performance, as shown in Figure 1A.
In accordance with the whole-brain cortical thickness results, the average normalized cortical volume was significantly smaller in elderly controls compared to middle-aged controls (Average Volume: elderly controls = 244.13 mm3; middle-aged controls = 306.43 mm3; p <.001).
SuperAgers Versus Middle-Aged Controls
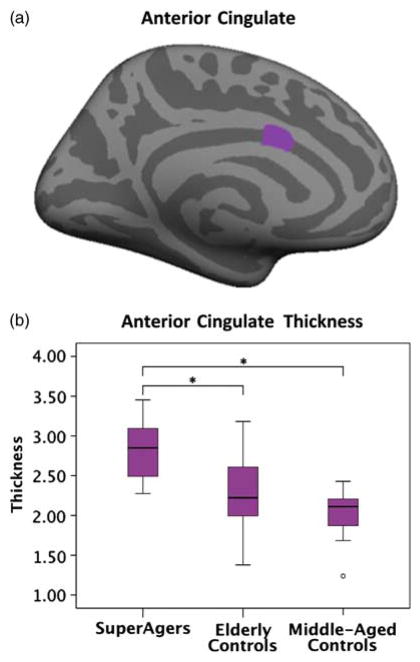
Surprisingly, the whole-brain cortical thickness analysis comparing SuperAgers and middle-aged controls did not reveal significant atrophy in the SuperAgers (Figure 1C). In addition, an area located in the left anterior cingulate (central estimated Talairach coordinates: −6, 10, 26) was thicker in SuperAgers than in middle-aged controls (http://surfer.nmr.mgh.harvard.edu/fswiki/CoordinateSystems). The average thickness of this region was quantified for each group and is shown in Figure 2.
Fig. 2.
Quantification of the left hemisphere anterior cingulate region by group. (a) Inflated medial surface of left hemisphere shows the anterior cingulate region, identified in the whole-brain group comparison (Figure 1B) as significantly thicker in SuperAgers than in middle-aged controls. (b) Boxplots of average left hemisphere anterior cingulate thickness data by group. * represents significant differences in average thickness at p <.05.
Results from the whole-brain normalized cortical volume analysis were similar to the whole-brain cortical thickness analysis, demonstrating no statistically significant differences between the SuperAgers and middle-aged controls (Average Volume: SuperAgers = 288.05 mm3; middle-aged controls = 306.43 mm3; p = .08).
SuperAgers Versus Elderly Controls
As expected from the results above, a comparison of healthy elderly controls and SuperAgers revealed areas of significant cortical thinning in the former group (Figure 1D). The cortical volume measures corroborated the thickness findings, showing SuperAgers had significantly larger normalized cortical volumes than their peer group of elderly controls (Average Volume: SuperAgers = 288.05 mm3; elderly controls = 244.13 mm3; p <.001).
Relationships Between Memory Performance and Brain Morphometry
Separate Pearson correlations were used to assess the relationship between episodic memory performance (i.e., RAVLT delay score) and cortical brain measures (i.e., normalized cortical volume and cingulate thickness) across the groups. Results showed a significant positive correlation between average normalized cortical volume and episodic memory performance (r = 0.532; p = .001) but no relationship between episodic memory performance and thickness of the cingulate.
DISCUSSION
We identified octogenarians and nonagenarians with superior memory function compared to their age-matched cognitively normal peers and found anatomic features associated with SuperAging that deviated from those associated with normal cognitive aging. The SuperAgers showed significantly greater cortical thickness and volume than their cognitively normal age-matched peers and showed no significant cortical atrophy when compared to younger, cognitively intact individuals 20–30 years younger (50- to 65-year-olds). These findings are remarkable given the numerous reports that grey matter loss is a common, if not universal, part of normal aging (Drachman, 2006; Fjell et al., 2006; Salat et al., 2004). Although the relationship between structural MRI-based cortical thickness measures and cellular morphology is not entirely understood, the lack of atrophy in SuperAgers may be taken as a proxy measure of preserved cortical neuronal integrity.
In addition to the absence of age-related cortical atrophy, our data also suggest that SuperAging may be associated with an unusual prominence of the cingulate cortex (Figure 2). At this point it is unclear whether SuperAgers were born with a particularly thick cortex or whether they resisted cortical change over time. Multiple lines of evidence converge toward the conclusion that the cingulate constitutes a critical site of trans-modal integration related to episodic memory, spatial attention, cognitive control, and motivational modulation (Mesulam, 2009; Mesulam, 1998). In our study, cingulate thickness was not directly correlated with memory performance, which is not surprising since the cingulate is not known as a primary component of the episodic memory circuitry. However, it is possible that the cingulate may mediate resistance of memory circuits to the deleterious processes of aging.
The posterior segment of the cingulate gyrus has recently received considerable attention in relationship to the patho-physiology and temporal evolution of AD. For example, cingulate hypometabolism as measured by metabolic positron emission tomography (PET) is one of the earliest correlates of neuronal dysfunction in AD (Minoshima et al., 1997) and in vivo amyloid imaging with PET shows early accumulation of amyloid in the cingulate gyrus of AD patients (Fripp et al., 2008; Sperling et al., 2009). Likewise, functional MR data suggest that the cingulate cortex is an important component of a large-scale resting-state network, which shows abnormalities early in the course of AD (Buckner et al., 2009; Sperling et al., 2009). Thus, normal cingulate function appears to be important for the integrity of multiple cognitive domains, and the SuperAgers’ superior episodic memory function may reflect, at least in part, the presence of a more extensively developed cingulate region that is also physiologically more resilient to age- and AD-related pathology.
While recording systematic and detailed medical and demographic histories of the SuperAgers we found no reason to suspect that they had unusually superior memory abilities when younger. In fact, their level of education was not abnormally high, only 4 of the 12 SuperAgers obtained a college degree. The findings in this cohort of cognitive SuperAgers provide ‘proof of concept’ that maintenance of superior memory together with cortical integrity is a biological possibility. Identifying the underlying factors that promote this trajectory of unusually successful cognitive aging may lead to novel insights for preventing age-related cognitive impairments or strategies for evading the more severe changes associated with Alzheimer’s disease.
Acknowledgments
This project was supported by a grant from The Davee Foundation and the Northwestern University Alzheimer’s Disease Core Center grant from the National Institute on Aging (AG13854). We are grateful to Rebecca Gavett and Katherine Reiter for their role in the neuropsychological testing of participants in this project.
A portion of the data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., as well as non-profit partners the Alzheimer’s Association and Alzheimer’s Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation.
Some data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni. loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.ucla.edu/wp-content/uploads/how_to_apply/ADNI_Authorship_List.pdf
Footnotes
The authors report no conflicts of interest.
References
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. The Journal of Neuroscience. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depp CA, Jeste DV. Definitions and predictors of successful aging: A comprehensive review of larger quantitative studies. The American Journal of Geriatric Psychiatry. 2006;14(1):6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Drachman DA. Aging of the brain, entropy, and Alzheimer disease. Neurology. 2006;67(8):1340–1352. doi: 10.1212/01.wnl.0000240127.89601.83. [DOI] [PubMed] [Google Scholar]
- Evans DA, Grodstein F, Loewenstein D, Kaye J, Weintraub S. Reducing case ascertainment costs in U.S. population studies of Alzheimer’s disease, dementia, and cognitive impairment-Part 2. Alzheimer’s & Dementia. 2011;7(1):110–123. doi: 10.1016/j.jalz.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Salat D, Quinn BT, Dale AM. Selective increase of cortical thickness in high-performing elderly - structural indices of optimal cognitive aging. Neuroimage. 2006;29(3):984–994. doi: 10.1016/j.neuroimage.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Fripp J, Bourgeat P, Acosta O, Raniga P, Modat M, Pike KE, Ourselin S. Appearance modeling of 11C PiB PET images: Characterizing amyloid deposition in Alzheimer’s disease, mild cognitive impairment and healthy aging. Neuroimage. 2008;43(3):430–439. doi: 10.1016/j.neuroimage.2008.07.053. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Heaton RM, Miller SW, Taylor MJ, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan battery: Demographically adjusted neuropsychological norms for african american and caucasian adults - professional manual. San Antonio, TX: Psychological Assessment Resources, Inc; 2004. [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Weiner MW. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. Journal of Magnetic Resonance Imaging. 2008;27(4):685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kaup AR, Mirzakhanian H, Jeste DV, Eyler LT. A review of the brain structure correlates of successful cognitive aging. The Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23(1):6–15. doi: 10.1176/appi.neuropsych.23.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Fischl B. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60(9):878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Defining neurocognitive networks in the BOLD new world of computed connectivity. Neuron. 2009;62(1):1–3. doi: 10.1016/j.neuron.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Annals of Neurology. 1997;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Randolph C. Repeatable battery for the assessment of neuropsychological status (RBANS) San Antonio, TX: The Pyschological Corporation; 1998. [Google Scholar]
- Rowe JW, Kahn RL. Successful aging. The Gerontologist. 1997;37(4):433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Saxton J, Ratcliff G, Munro CA, Coffey EC, Becker JT, Fried L, Kuller L. Normative data on the Boston Naming Test and two equivalent 30-item short forms. The Clinical Neuropsychologist. 2000;14(4):526–534. doi: 10.1076/clin.14.4.526.7204. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Rey Auditory Verbal Learning Test: A handbook. Torrance, CA: Western Psychological Services; 2004. [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Wicklund AH, Salmon DP. The Neuropsychological profile of Alzheimer’s disease. In: Dennis EM, Selkoe J, Holtzman D, editors. The biology of Alzheimer’s disease. New York: Cold Spring Harbor Laboratory Press; 2011. pp. 25–42. [Google Scholar]