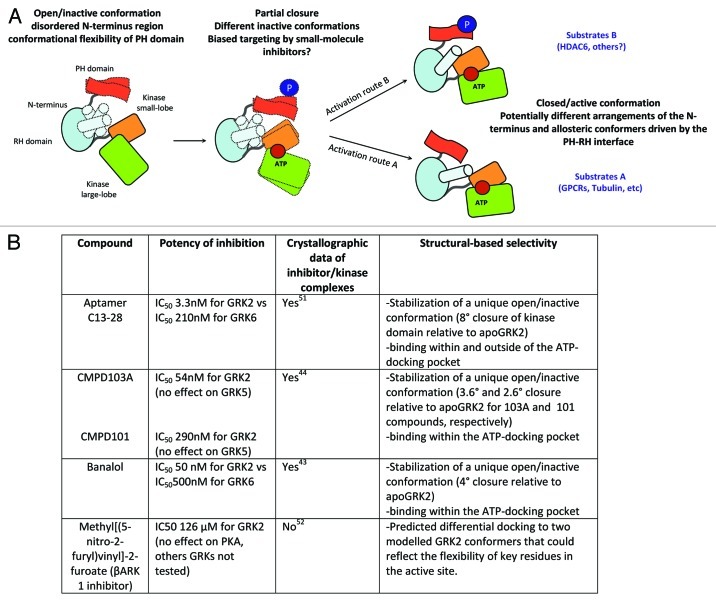
Figure 2. (A) Proposed model for the potential occurrence of different active/inactive conformers at the GRK2 kinase domain. The full kinase domain closure induced by the presence of ATP and the substrate is needed to render GRK2 catalytically competent. Inactive GRK2 adopts an open conformation with a disordered N-terminus (indicated by dotted shapes) and poor arrangement of the AST (active site tether) region that passes over the small and large kinase lobes. Both regions become increasingly ordered as GRK2 approaches its active/closed conformation. Moreover, kinase closure is allosterically induced/ influenced by the binding of a variety of substrates (GPCRs, cytosolic proteins) that would establish specific contacts with GRK2. In addition, the C-terminal PH domain might participate in the allosteric activation of GRK2 due to its interaction with the RH domain, and this interface can be altered by the docking of phospholipids, Gβγ subunits or posttranslational modifications. We hypothesize that the extent of GRK2 phosphorylation at Ser670 may alter the allosteric communication relayed by the PH-RH axis, what might route the activation process through slightly different conformations of the active site, resulting in distinct substrate selectivity and potential differential inhibitor sensitivity (see text for details). (B) Crystallographic analysis of several GRK2-inhibitor complexes compared with the GRK2-ATP complex (apoGRK2) indicate that selective inhibitors could “freeze” the kinase at different unique inactive conformations that might arise during closure of the kinase domain. It must be note that for crystallization purposes, a mutant Ser670→Ala670 variant of GRK2 has been routinely used in these studies.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.