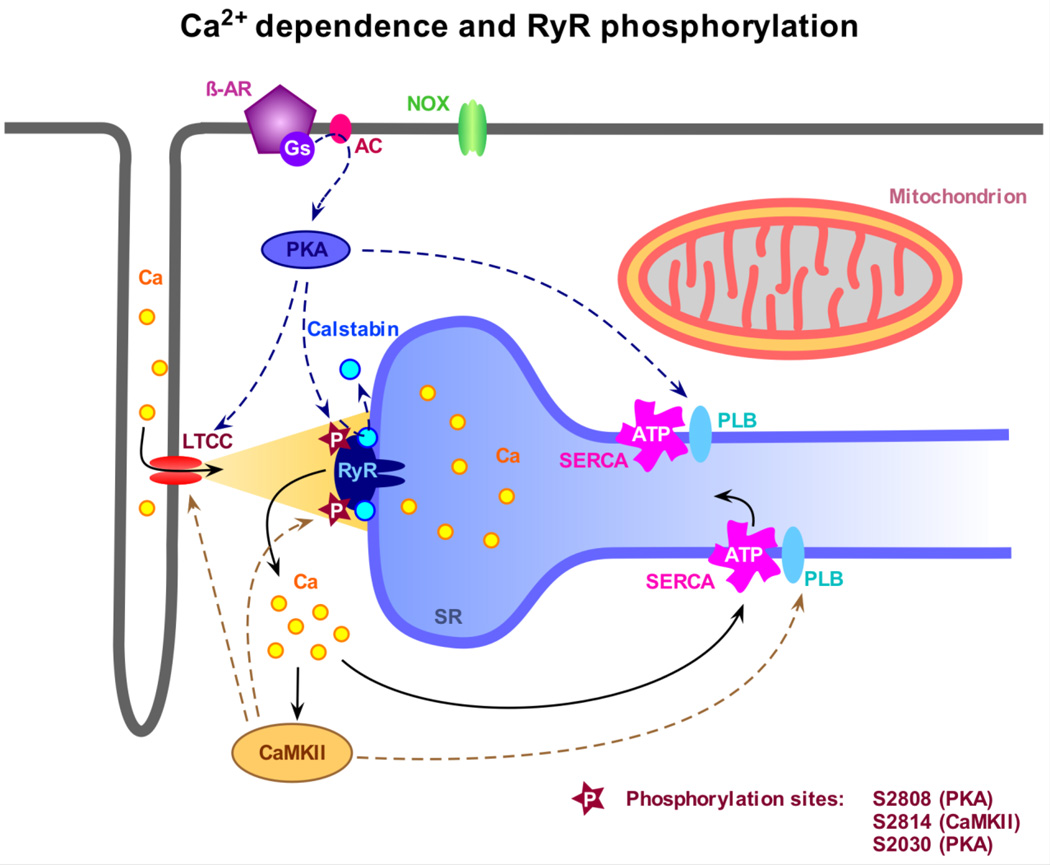
Fig. 1.
Modulation of the ryanodine receptor (RyR) by Ca2+ and phosphorylation. Ca2+ influx via the L-type Ca channel (LTCC) activates the RyR and triggers Ca2+ release from the sarcoplasmic reticulum (SR), a process referred to as Ca2+ induced Ca2+ release or CICR, leading to myocyte contraction. The levels of free cytosolic Ca2+ are tightly regulated by the SR Ca2+ ATPase (SERCA) and the sarcolemmal Na+-Ca2+ exchanger (not indicated). After CICR and contraction, the Ca2+ store is refilled by pumping Ca2+ back into the SR thereby re-establishing diastolic Ca2+ levels. The sensitivity of RyR toward activating Ca2+ is modulated by phosphorylation. Stimulation of the β1-adrenoreceptor (β-AR) leads to Gs-protein-mediated activation of adenylyl cyclase (AC) and further cAMP-dependent activation of PKA. PKA can directly phosphorylate RyR at several phosphorylation sites, presumably at S2808, possibly inducing dissociation of calstabin 2, and at S2030, but also modulates the LTCC and SERCA function, the latter by phosphorylation of phospholamban (PLB). Increased cytosolic Ca2+ levels activate CaMKII, which directly phosphorylates RyR at S2814. Similar to PKA, CaMKII also phosphorylates PLB and the LTCC leading to global changes in myocyte Ca2+ homeostasis.