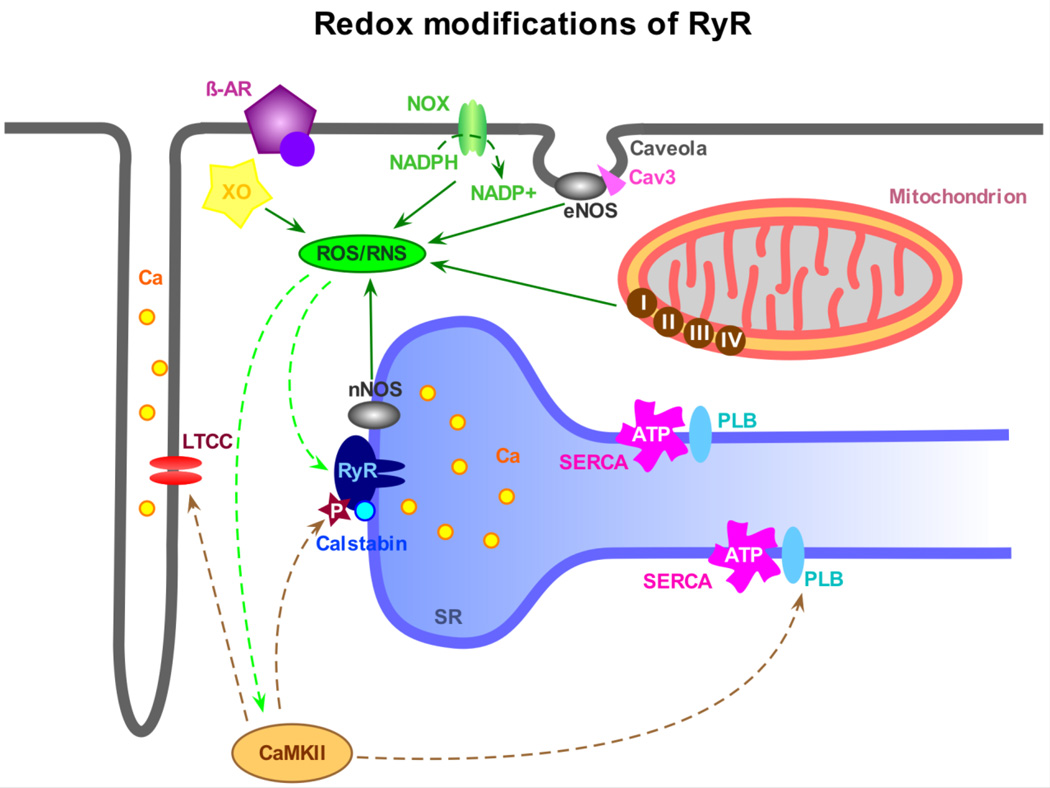
Fig. 2.
Redox-modifications of RyRs. Changes in the redox potential of the myocyte have been shown to have a serious influence on protein function, especially at the level of the RyR. The main sources for the production of reactive oxygen species (ROS) in cardiomyocytes are the sarcolemmal NADPH oxidase (NOX), the xanthine oxidase (XO) and the mitochondrial electron transport chain (complex I through IV). ROS can glutathionylate free cysteine residues on the RyR and also act in an indirect way via CaMKII activation and subsequent RyR phosphorylation. Nitric oxide synthases (NOS) are mainly responsible for the production of nitric oxide (NO) and reactive nitrogen species (RNS). In cardiomyocytes, sarcolemmal endothelial NOS (eNOS), which co-localizes with caveolin-3 (Cav3) in caveolae, and RyR-associated neuronal nNOS are primarily responsible for the production of NO, causing S-nitrosation at free thiol groups of the RyR and many other proteins. Most likely, these mechanisms work synergistically and induce parallel modifications of RyR function.