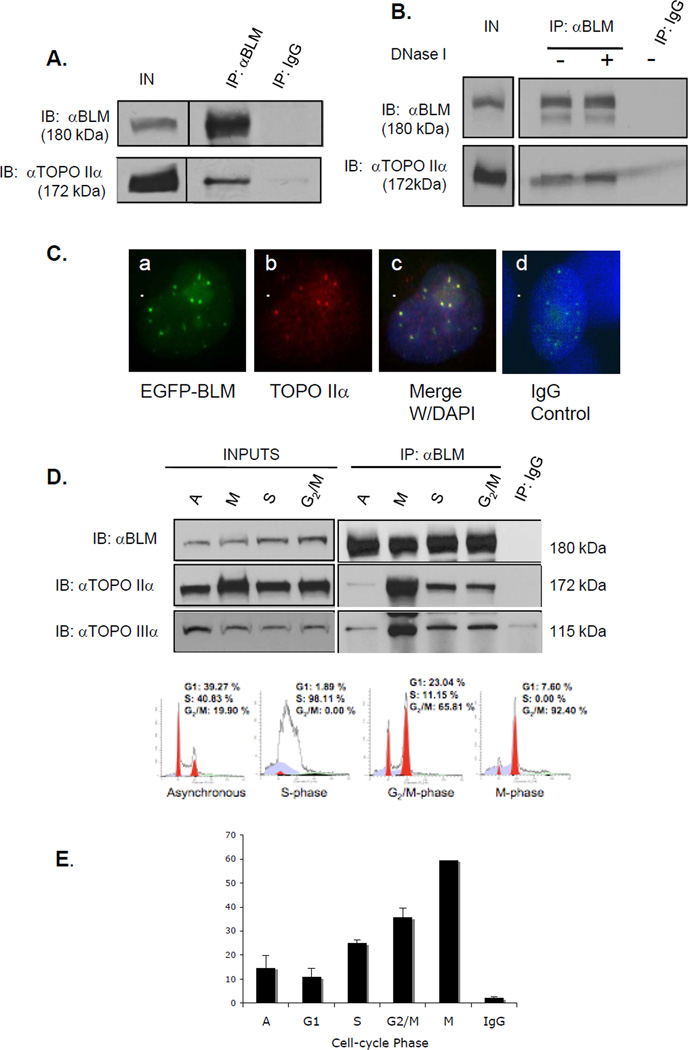
Figure 1. BLM and topoisomerase IIα associate and co-localize in human cells.
(A) BLM was immunoprecipitated from 293T nuclear extracts with a rabbit anti-BLM antibody. Rabbit IgG was used as a control. Proteins were separated using SDS-PAGE and analyzed by western blotting with anti-BLM or anti-topoisomerase IIα antibody. (B) 293T cells were transfected with pEGFP-BLM and BLM immunoprecipitated as in A, with or without DNase I. (C) MCF7 cells were transfected with pEGFP-BLM, fixed and stained with an anti-topoisomerase IIα antibody and DAPI for nuclear staining. Panel a. shows EGFP-BLM-positive foci; panel b. shows topoisomerase IIα staining; panel c. shows merged images and DAPI staining; panel d. shows staining with rhodamine-labeled mαIgG and DAPI staining. (D) Nuclear extracts from synchronized 293T cells were used in immunoprecipitations. Western blotting with an anti-topoisomerase IIIα antibody was used as a positive control. Inputs are shown in the left panel; immunoprecipitations in the right panels. The bottom panel shows FACS analysis of representative cell populations used for immunoprecipitations. (E) The percentage of BLM foci that co-localize with topoisomerase IIα foci during cell cycle phases in HCT 116 cells is shown graphically.