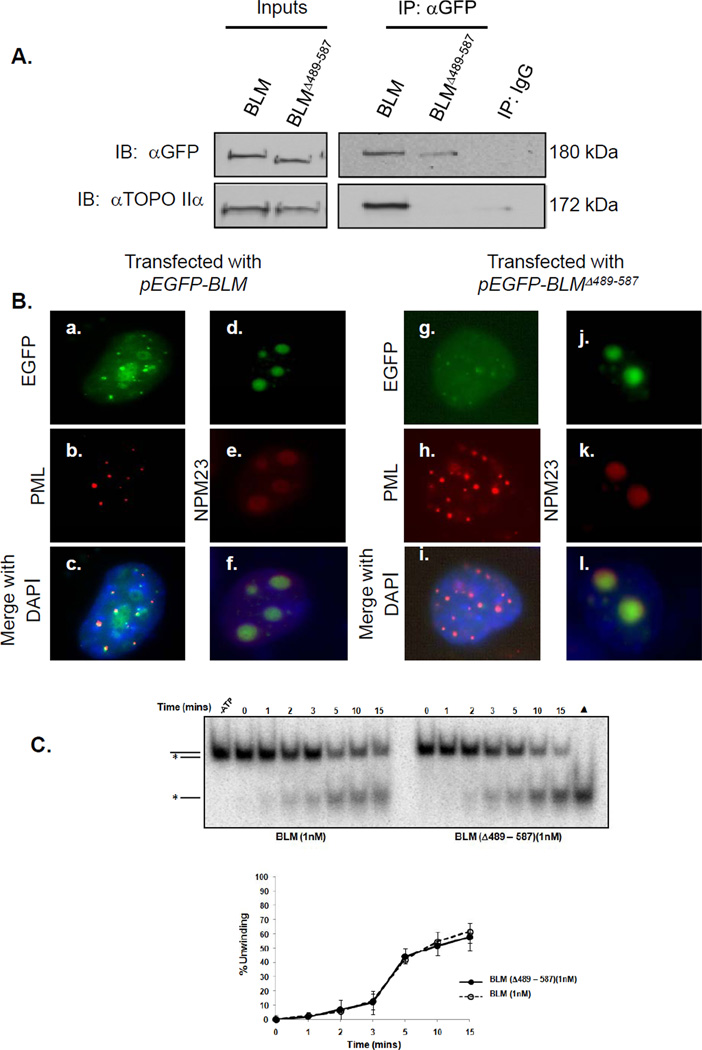
Figure 4. Amino acids 489-587 in BLM are necessary for interaction with topoisomerase IIα but are not required for localization or helicase activity.
(A) 293T cells were transfected with pEGFP-BLM or pEGFP-BLMΔ489-587. Post-transfection, wild-type and mutant BLM proteins were immunoprecipitated from nuclear extracts with mouse anti-GFP antibody or mouse IgG control, separated using SDS-PAGE and analyzed by western blotting with anti-GFP or anti-topoisomerase IIα antibody. (B) GM08505 BS cells were transfected with pEGFP-BLM or pEGFP-BLMΔ489-587. Cells were stained with anti-PML antibodies and anti-NPM23 antibodies to recognize PML bodies and the nucleolus, and DAPI for nuclear staining. EGFP-BLM or EGFP-BLMΔ489-587 co-localization with PML and NPM23 is shown. (C) Helicase activities of BLM and BLMΔ489-587 using 3’ DNA overhang substrates are equivalent. BLM proteins were incubated with 2 fmoles of substrate; resulting products were resolved by PAGE and visualized by autoradiography (top). A heat-denatured control (▲) shows migration of the released radio-labeled strand (grey). The broken line represents unwinding by 1 nM BLM. The solid line represents unwinding by 1 nM BLMΔ489-587.