Abstract
A dendrimer-based sandwich type enzyme-linked immunosorbent assay (ELISA) was developed for the improved detection of recombinant human tumor necrosis factor-alpha (TNF-α) for early diagnosis of perinatal diseases. Hydroxyl-terminated generation four poly(amidoamine) dendrimer (G4-OH) was used for the development of a solid phase bio-sensing platform. The surface of the ELISA plate was modified with polyethylene-glycol (PEG) and thiol-functionalized G4-OH was immobilized on the PEG-functionalized plate. A capture antibody was oxidized and covalently immobilized onto the dendrimer-modified ELISA plate, which provides favorable orientation for the antigen binding sites towards the analyte. The dendrimer-modified plate showed enhanced sensitivity, and the detection limit for TNF-α was found to be 0.48 pg mL−1, which is significantly better than the commercially available ELISA kit. The selectivity of the dendrimer-modified ELISA plate was further evaluated with a mixture of cytokines, which showed results for similar to that of TNF-α alone. The modified plate provides a greater opportunity for the detection of a wide range of cytokines and biomarkers.
Keywords: PAMAM dendrimer, TNF-α, ELISA, Dendrimer biosensor
1. Introduction
Intra-amniotic infection/inflammation (IAI) is one of the most important causes of preterm birth (PTB) and perinatal morbidity. The relationship between IAI and preterm labor has been well established.1 It accounts for 75% of perinatal mortality and 50% of perinatal morbidity.2,3 Early detection of this infection is challenging due to the sub-clinical nature of the disease.4,5 Fever, uterine tenderness and fetal tachycardia occur late in pregnancy and present in small portion (12.5%) of the women with microbiological evidence of infection.6,7 An elevated level of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, and MMP-8) are found in the infected intra-amniotic fluid, which can serve as biomarkers for early detection.8,9 The development of sensitive and specific diagnostic device that will enable an early detection and treatment to prevent fetal damage would be of great interest. The importance of detection of these pro-inflammatory cytokines is also enhanced by their association with a variety of diseases such as pulmonary and neurodegenerative diseases, where inflammation plays a central role in the pathogenesis of the disease. Elevated levels of TNF-α are found in the cerebrospinal fluid of patients suffering from Alzheimer’s disease10, Parkinson’s disease11, and multiple sclerosis12, and in the airways and blood of patients with Chronic Obstructive Pulmonary Disease (COPD).13,14 Therefore, improved detection of this important biomarker can help in diagnosis of many diseases.
TNF-α is a pro-inflammatory cytokine that regulates the immune system inducing inflammation and apoptosis in many organs of the body. In the brain, overexpression of TNF-α in microglia cells causes neural death which is linked with neurological disorders. An increased level of TNF-α is also associated with pre-term birth and cerebral palsy.15 Therefore, measuring the level of TNF-α in amniotic fluid can be essential for an understanding of the mechanism of pre-term birth and its prevention. Enzyme-linked immunosorbent assay (ELISA) is the most common method used to determine the concentration of TNF-α. Other detection methods include radioimmunoassays (RIA)16, bioassays17, chemiluminescence18 and chemiluminescence imaging19, matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS)20, immuno-PCR21 and immuno-PCR assay22, fluorescence immunoassay23 and electrochemical immunosensor.24 Most methods employ commercially available ELISA plates, which show relatively high background signal due to non-specific protein adsorption. Our goal is to develop a diagnostic device that will detect the presence of TNF-α at very low levels by improving its sensitivity and specificity. Development of a dendrimer-templated sensor surface that minimizes the non-specific protein adsorption, and leads to appropriate presentation of the anti-body binding sites (analyte) through dendrimer conjugation that increases ligand capture efficiency, is expected to improve the sensitivity and selectivity.25 The dendrimer-based assay in present study has a detection limit superior to that of other previously reported TNF-α assays except that obtained by Hun et al. where fluoroimmunoassay method was used.23 In developing an immunosensor, the antibodies should be immobilized onto a substrate at high density with uniform distribution followed by retaining their specific antigen-binding activities, and finally maintaining accessibility to the antigens.26 Multifunctional dendrimers are employed in this work.
Dendrimers are well-defined, stable and versatile nanoscale polymers can be closely packed on the substrate surface, and have multiple branch ends available for further conjugation.27-29 They have the characteristics to be used as the next generation of biosensors.30,31 Dendrimers have been recently reported to significantly increase the binding ability and homogeneity in DNA microarray analysis.32-34 Dendrimers are also utilized in electrochemical immunosensor,35,36 biosensor-based encapsulated platinum nanoparticles,37,38 regenerable affinity-sensing surfaces39 and surface plasmon resonance biosensors.40 The application of dendrimer has been extended to preparation of metal-dendrimer nanocomposites using PAMAM dendrimers with various terminal groups.41-43 Generation 4.5 carboxyl-terminated PAMAM dendrimers were shown to have lower the nonspecific cellular protein adsorption due to their negatively charged surface that leads to reduced protein interactions and at the same time enhanced the antibody binding properties because of the presence of higher number of carboxyl groups at their surface.26
Recently we have reported a highly sensitive dendrimer-based biosensing platform for IL-6 and IL-1β cytokines detection in which PEGylated ELISA plate was modified using amine terminated PAMAM dendrimer.44 In continuing our effort, we have developed a solid phase biosensing platform using generation four hydroxyl terminated PAMAM dendrimer (G4-OH) for TNF-α cytokine detection. The synthesis, characterization, fabrication and functional evaluation of this biosensing platform for TNF-α detection are described in this paper. The resulting dendrimer functionalized plate was employed as template to immobilize the anti-human TNF-α antibody and the performance was evaluated by the detection of TNF-α using TMB detection method. The dendrimer-based assay was compared to commercially available TNF-α kit.
2. Experimental Methods
2.1 Chemicals and Materials
Hydroxyl-terminated generation four poly(amidoamine) dendrimer (G4-OH) was purchased from Dendritech Inc. (Midland, MI USA). N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDC), 4-(dimethylamino)pyridine (DMAP), N-succinimidyl-3-(2-pyridyldithio)-propionate (SPDP) and MALDI-TOF mass reference standards (all from Sigma-Aldrich), trifluoroacetic acid (TFA) (EMD Chemicals), DMSO-d6 (Cambridge Isotope Laboratories), anhydrous DMSO and dichloromethane (DCM) (Acros Organics, USA) and N-(ε-maleimidocarproic acid) hydrazide (EMCH) (Pierce Chemicals), were purchased at the highest purities available. Hydoxyl terminated polyethylene glycol (NH2-PEG-OH, 2.0 kDa) and maleimide terminated PEG (NH2-PEG-Mal, 3.4 kDa) were obtained from JenKem Technology (Beijing, China) and Creative PEGworks (Winston Salem, USA) respectively. Regenerated cellulose (RC) dialysis membrane (molecular weight cut-off 1000 Da) was obtained from Spectrum Laboratories. Recombinant human TNF-α, monoclonal TNF-α anti-human antibody, biotinylated anti-human TNF-α antibody, streptavidin-HRP (DY998), reagent diluent (DY995), substrate reagent (TMB, DY999), stop solution (DY994) and wash buffer (WA126) were all purchased from R&D Systems. ELISA plates were purchased from Corning (high bind plate).
2.2 NMR Spectra Analysis
NMR spectra were recorded on a Varian INOVA 400 spectrometer using DMSO-d6 as solvent. Proton chemical shifts are reported in ppm (δ) and tetramethylsilane (TMS) used for internal standard.
2.3 MALDI-TOF mass
Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectra were recorded on a Bruker Ultraflex system. Cytochrome C (MW 12,361 g mol−1), and Apomyoglobin (MW 16,952 g mol−1) were used as external standards. A dendrimer solution was prepared by dissolving 2 mg of dendrimer in 1 mL of DMSO. The matrix solution was prepared by dissolving 20 mg of trans-3-indoleacrylic acid matrix in 1 mL of the 1:1 mixture of deionized water and acetonitrile (0.1% TFA). Analytical samples were prepared by mixing 10 μL of dendrimer solution with 100 μL of matrix solution, followed by deposition of 1 μL of sample mixture onto a 384-well aluminum plate. This mixture was allowed to air dry at room temperature. All data processing was performed using Bruker Daltonics flex Analysis software.
2.4 High Performance Liquid Chromatography (HPLC)
HPLC characterization was carried out using Waters HPLC instrument. Two pumps, an auto-sampler and dual UV detector are interfaced to Breeze software. The HPLC chromatogram was monitored at 210 and 238 nm simultaneously using the dual UV absorbance detector. H2O:ACN (0.14% TFA) was used as mobile phase. Both phases were freshly prepared, filtered, and degassed prior to use. Symmetry300 C18 reverse-phase column (5 μm particle size, 25 cm × 4.6 mm length × I.D.) equipped with a Supelguard Cartridges (5 μm particle size, 2.0 cm ×3.9 mm length × I.D.) was used for characterization of the conjugates. HPLC analysis was done using 90:10 to 30:70 (H O:ACN) gradient flow in 30 min with flow rate of 1 mL min−1.
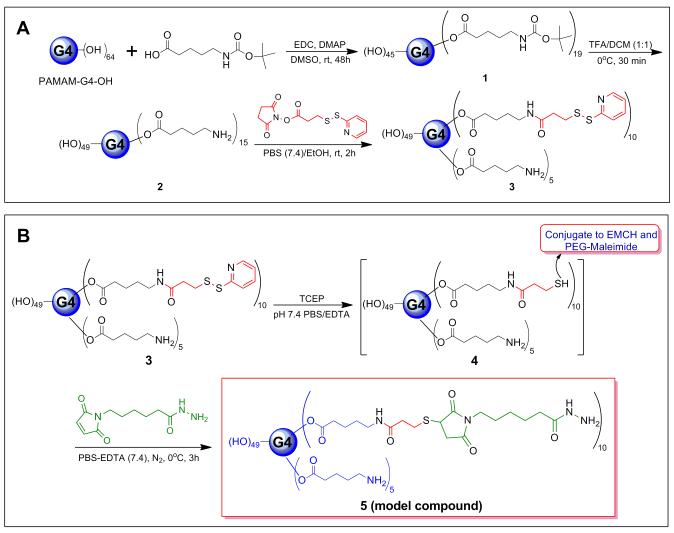
2.5 Synthesis of Bifunctional PAMAM G4-OH Dendrimer (2)
The 5-(Boc-amino)valeric acid (228.2 mg, 1.0504 mmole) was dissolved in DMSO (10 mL) and EDC (603.8 mg, 3.15 mmole) and DMAP (12 mg, 0.0982 mmole) were added to it under nitrogen atmosphere. The mixture was allowed to stir for 1 h at room temperature and G4-OH (500 mg, 0.0350 mmole) dissolved in DMSO (5 mL) was added to the reaction mixture. The reaction mixture was stirred for 48 h at room temperature. The resulting solution was dialyzed extensively with DMSO (dialysis membrane of molecular weight cutoff = 1000 Da) for 24 h, and with deionized water for 6 h. The resultant reaction mixture was then lyophilized, yielding 445.0 mg of intermediate 1 (G4-OH-Link-Boc). The intermediate 1 contains Boc protected amino groups on the surface. 1H NMR (DMSO-d6, 400 MHz): δ ppm 8.04 (bs, CO-NH, Boc), 7.92 (bs, CO-NH, G4-OH), 7.77 (bs, CO-NH, G4-OH), 4.69 (bs, OH, G4-OH), 3.96 (m, CH2OCO), 3.37-2.18 (986H, m, aliphatic protons of G4-OH and CH2 of linker), 2.01 (m, CH2, methylene protons of linker), 1.45 (m, 2 × CH2, methylene protons of linker), 1.34 (s, 3 × CH3, methyl groups of Boc). The deprotection of Boc-protected amino groups was done by adding 6.0 mL of TFA:DCM (1:1) to 445.0 mg of intermediate 1 in a round bottom flask at 0°C. The reaction was carried out for 1 h at 0 °C, and then the solvent was removed at reduced pressure. The mixture was re-dissolved in DMSO and dialyzed with DMSO for 24 h and deionized water for 6 h. The reaction mixture was lyophilized yielding 298 mg of bifunctional dendrimer 2. 1H NMR (DMSO-d6, 400 MHz): δ ppm 7.92 (bs, CO-NH, G4-OH), 7.77 (bs, CO-NH, G4-OH), 4.69 (bs, OH, G4-OH), 3.99 (t, CH2OCO), 3.40-2.47 (986 H, m, aliphatic protons of G4-OH and CH2 of linker), 2.31 (m, CH2, methylene protons of linker), 1.53 (m, 2 × CH2, methylene protons of linker).
2.6 Synthesis of Bifunctional PAMAM G4-OH-PDP Dendrimer (3)
The bifunctional dendrimer 2 (150.0 mg, 0.00937 mmole) was dissolved in 9 mL of PBS buffer (pH 7.4) and SPDP (87.0 mg, 0.2798 mmole) dissolved in 3.5 mL ethanol was added. The reaction mixture was stirred for 2 h at room temperature under nitrogen condition. The resulting solution was dialyzed extensively with water (dialysis membrane of molecular weight cutoff = 1000 Da) for 24 h and lyophilized to get 90.0 mg of intermediate 3. 1H NMR (DMSO-d6, 400 MHz): δ ppm 8.43 (d, 1H, pyridyl H), 8.12 (bs, CO-NH, linker, G4-OH), 7.98 (bs, CO-NH, G4-OH), 7.81 and 7.73 (m, pyridyl Hs), 7.22 (t, 1H, pyridyl H), 4.75 (bs, OH, G4-OH), 3.98 (m, CH2OCO), 3.51-2.08 (m, 990H, aliphatic protons of G4-OH, CH2 of linker, and CH2 of PDP.), 2.04 (m, CH2, methylene protons of linker), 1.48 (m, CH2, methylene protons of linker), 1.36 (m, CH2, methylene protons of linker). MALDI-TOF (pos) m/z 17141.1.
2.7 Synthesis of EMCH Functionalized PAMAM G4-OH-PDP Dendrimer (5)
The PDP-functionalized bifunctional dendrimer 3 (2.4 mg, 140 nmole) was dissolved in 1 mL of PBS/EDTA buffer (pH 7.4, 5 mmole EDTA) and 0.80 mg of TCEP (2.80 μmole, 20 equiv.) dissolved in 1 mL of PBS/EDTA buffer was added to it. The reaction mixture was stirred for 15 min at room temperature under N2 to get the free thiolated conjugate 4. The solution was cooled down to 0°C and 0.72 mg of EMCH (2.10 μmole, 15 equiv.) in 1 mL of PBS buffer was added and stirred for 3 hrs at room temperature under N2. The resulting solution was extensively dialyzed with water (membrane MW cutoff = 1000 Da) for 24 h and lyophilized to give conjugate 5 as a pale yellow semi-solid. 1H NMR (DMSO-d6, 400 MHz): δ ppm 8.90 (s, 1H, hydrazide amide NH), 7.94 (bs, CO-NH, linker, G4-OH), 7.82 (bs, CO-NH, G4-OH), 4.8 (bs, OH, G4-OH), 3.95 (m, CH2OCO), 3.50-2.00 (m, 997H, aliphatic protons of G4-OH, CH2 of linker, CH2CH2SCHCH2, NCH2, and NH2), 1.89 (m, CH2, methylene protons of linker, and CH2NHNH2), 1.58-1.32 (m, CH2, methylene protons of linker and CH2NHNH2of EMCH), 1.30-1.05 (m, CH2, methylene protons of linker and EMCH).
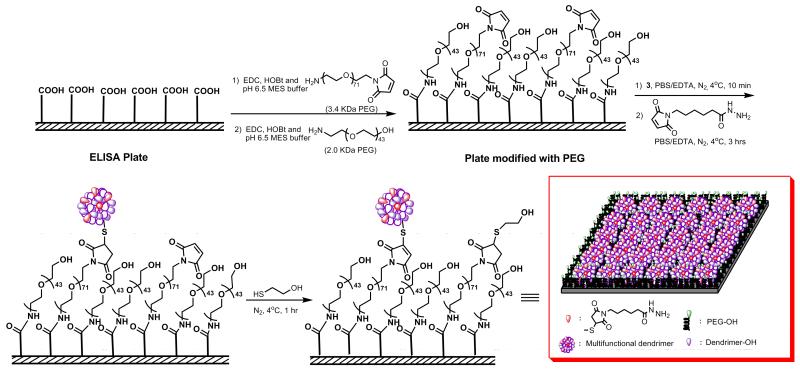
2.8 Modification of ELISA Plate with PEGs
Solutions of EDC and HOBt in MES buffer (pH 6.5, 1 mg mL−1) were prepared, from which 75 μL of EDC solution and 60 μL of HOBt solution were added to each well of ELISA plate (391 nmol/well). NH2-PEG-Mal (MW=3.4 kDa, 10 μL) dissolved in MES buffer (pH 6.5, 12.52 μg mL−1) was added to each well (7.4 pmole/well) and incubated for 30 min at room temperature. Then 30 μL of NH2-PEG-OH (MW=2 kDa) dissolved in MES buffer (pH 6.5) was added to each well (5.81 mg/well). The plate was incubated for 8 h at room temperature with constant shaking. Then ELISA plate was washed with PBS buffer (pH 7.4) three times and with deionized water (3 times). The PEG modified plate was dried under nitrogen and stored at −20°C.
2.9 Conjugation of Dendrimer 3 to PEGylated ELISA Plate
The PDP-functionalized dendrimer conjugate 3 (1.2 mg, 70 nmole) was dissolved in 1 mL of PBS/EDTA buffer (pH 7.4, 5 mmole EDTA), and 0.4 mg of TCEP (1.4 μmole, 20 equiv.) dissolved in 0.5 mL of PBS/EDTA buffer was added. The reaction mixture was stirred for 15 min at room temperature under N2 to get the free thiolated conjugate 4. The solution was transferred to a glove box and cooled down to 0°C. EMCH (0.16 mg, 0.49 μmole, 7 equiv.) in 0.5 mL of PBS buffer was added to the solution and incubated for 20 min at 0°C. Then 10 μL of this reaction mixture was diluted to 31.5 mL with PBS buffer (pH 7.4) and 270 μL of diluted solution was added to each well of pegylated ELISA plate (3 pmole/well). The plate was incubated for 3 h at room temperature in glove box with constant shaking. 1 μL of 2-mercaptoethanol dissolved in 10 μL of PBS (pH 7.4) was added to each well and incubated for 1 h at room temperature. The ELISA plate was washed with PBS buffer (pH 7.4) four times and with deionized water four times, dried under nitrogen and stored at −20°C.
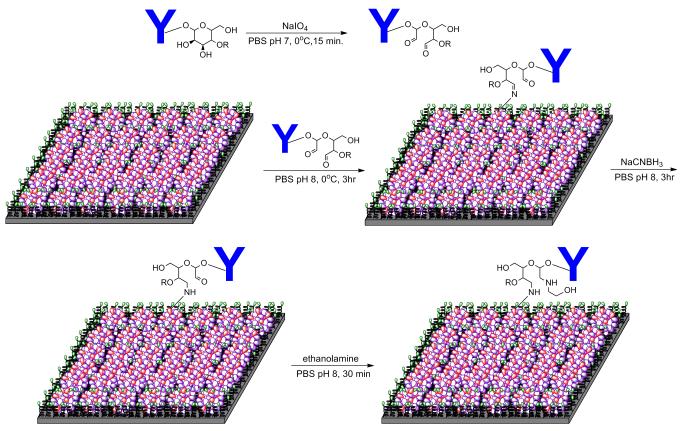
2.10 Oxidation of Monoclonal Anti-Human TNF-α/TNFSF1 Antibody (MAB610) and Its Immobilization to Dendrimer Modified ELISA Plate
MAB610 antibody (500 μg) was dissolved in 1 mL of PBS buffer (pH 7.0) and 100 μL of NaIO4 solution in PBS (pH 7.0) was added at 4°C (5 μg NaIO4 per 50 μg MAB610). The reaction mixture was kept at 4°C for 15 min in dark, transferred to Amicon centrifuge filter tube and centrifuged for 30 min (2500 rpm) at 4°C. The concentrated sample (approx. 60 μL) was diluted with PBS buffer (pH 7.4) to 1 mL and was centrifuged in previously equilibrated PD-10 column with PBS (pH 7.4) at 1000 G for 2 min (4°C). The concentrated antibody was diluted to 11 mL with PBS buffer (pH 8.0) and 270 μL was added to each well of dendrimer modified ELISA plate (3 μg/well). The ELISA plate was incubated for 3 h at 4°C with continuous shaking. After that, 10 μL of NaCNBH3 solution in PBS buffer (pH 8.0) (1mg mL−1) was added to each well and incubated for 1 h at 4°C with shaking. The ethanolamine solution (10 μL) in PBS buffer (pH 8.0) (1μL mL−1) was added to each well and incubated for 30 min at 4°C. Antibody immobilized dendrimer modified ELISA plate was then washed with wash buffer (WA126) seven times. Reagent diluents (300 μL) was added to each well and incubated overnight at 4°C. The plate was washed with wash buffer (7 times, 300 μL/well) for next step.
2.11 Assay Procedure
Twelve different dilutions (concentrations from 460-0 pg mL−1) of TNF-α in reagent diluents were prepared and 100 μL of each dilution were added per well in triplicate and incubated for 2 h at room temperature. The plate was washed with wash buffer (300 μL/well) seven times and 100 μL of biotinylated anti-human TNF-α detection antibody (BAF 210) dissolved in reagent diluents (1 μg mL−1) was added to each well. The plate was sealed and incubated for 2 h at room temperature and was washed with wash buffer again. Streptavidin-horseradish peroxidase (100 μL) dissolved in reagent diluents was added to each well and incubated for 30 min at room temperature. The plate was washed again with wash buffer and 100 μL of substrate (tetramethylbenzidine) solution was added to each well and incubated 30 min in dark at room temperature; a blue color was obtained. Then 50 μL of stop solution (2N H2SO4) was added to each well to stop the reaction and a yellow color was formed. The UV absorbance was measured immediately using a plate reader (Molecular Devices, SpectraMax M2) at 450 and 570 nm. Dual wavelength was chosen to reduce optical interference caused by scratches, fingerprints etc. that absorb light equally at both wavelengths.
3. Results and Discussion
3.1 Synthesis of PDP Functionalized PAMAM G4-OH Dendrimer
Previously published results indicate that many commercially available ELISA plates have higher background due to non-specific protein adsorption.45 In order to reduce the non-specific protein adsorption and to improve the specificity and sensitivity of immunoassay technique, the hydroxyl-terminated G4-PAMAM dendrimer was used to develop an assay platform for TNF-α cytokine detection. A bifunctional dendrimer was prepared by modifying approximately 15 hydroxyl terminal groups of generation four PAMAM dendrimer (G4-OH) to reactive amine for this platform.46,47 In brief, the G4-OH was reacted with 5-Boc-amino-valeric acid to get Boc protected amine groups followed by deprotection with trifluoroacetic acid to generate a bifunctional dendrimer (2) with free amine groups on the dendrimer (Figure 1A). The resulting bifunctional dendrimer was conjugated with N-succinimidyl-3-(2-pyridyldithio)-propionate (SPDP) through amide bond to get Pyridyldithio-propionate (PDP)-functionalized G4-OH, i.e., G4-OH-PDP, 3 (Figure 1A). PDP-functionalized thiol protected group is essential on the surface of the dendrimer which will be immobilized on PEGylated ELISA plate. The conjugate 3 was characterized by HPLC, 1H NMR, and MALDI-TOF mass. A peak at 9.55 min in the HPLC trace confirms the formation of the conjugate and it is broad due to its larger size and lower polarity compared to the starting dendrimer, G4-OH (Figure S-1A). The number of PDP groups attached to G4-OH was determined by comparing amidic protons of dendrimer to pyridyl protons of PDP groups by proton integration method in NMR analysis (Figure S-1B). The integration revealed that 10 molecules of PDP were attached to the surface of the dendrimer. A doublet at 8.43, two multiplets at 7.81 and 7.73, and a triplet at 7.22 ppm along with dendrimer peaks also confirm the formation of the PDP conjugate 3. This was in good agreement with MALDI-TOF mass analysis which showed that approximately 10.7 molecules of PDP were reacted to the dendrimer (Figure S-1C), as shown in the equation below.
Figure 1.
(A) Preparation of bifunctional dendrimer (2), and PDP-functionalized PAMAM G4-OH dendrimer (G4-OH-PDP, 3). (B) Synthesis of model compound 5. G4-OH-PDP (3) was reduced by TECP in PBS buffer resulting free thiol intermediate, 4 which again reacted with EMCH to get model compound (5) under nitrogen at 0°C.
A model compound 5, was prepared to validate the reactivity of the PDP-functionalized conjugate to the PEGylated ELISA plate (Figure 1B). The disulfide bond of PDP groups of conjugate 3 was reduced by TCEP to thiol quantitatively.48,49 The resulting intermediate 4 was reacted with EMCH in situ which is responsible for antibody capturing. The remaining thiol groups were required for immobilization of dendrimer to maleimide groups of PEGylated ELISA plate. Therefore, thiol groups in intermediate 4 were conjugated with EMCH to give hydrazide functionalized dendrimer 5. To prevent possible side reaction, the thiol-maleimide conjugation reaction was carried out at 0°C. The structure of conjugate 5 was confirmed by 1H NMR, which showed a characteristic hydrazide amide proton peak at 8.90 ppm (Figure S-2). The absences of any peak corresponding to the pyridyl protons in 1H NMR, confirms the formation of 5.
3.2 Immobilization of ELISA Plate with PEG and Dendrimer
To overcome the non-specific protein adsorption in commercially available ELISA plates, we modified our plates with polyethylene glycol (PEG). It has been reported that the non-specific adsorption of proteins decreases with increasing molecular weight of PEG chain. On the other hand, by increasing molecular weight of PEG, the tethered chain density decreases due to the exclusion volume of each chain on the surface.50 PEG-maleimide (NH2-PEG-Mal) and PEG-hydroxy (NH2-PEG-OH) were linked to the carboxylic acid functionalized 96 well polystyrene plate using EDC and HOBt under mild conditions to minimize the ring opening side reaction44 (Figure 2). The resulting HOBt activated carboxylic groups could be hydrolyzed under desired mild conditions which minimized the ring-opening side reaction of NH2-PEG-Mal.51 PEG-maleimide and PEG-hydroxy were co-immobilized to improve the non-fouling character of the surface and also cover the ‘defects’ on the polystyrene plate.45 The maleimide groups of NH2-PEG-Mal were responsible for immobilization of the dendrimer on the ELISA plate. Two equivalents of NH2-PEG-Mal were reacted with activated carboxylic acid groups, taking into consideration, i) the dendrimer graft density, ii) the yield of the amidation reaction, and iii) the reduced reactivity of thiol-maleimide conjugation reaction in the presence of TCEP.48 Thus, NH2-PEG-Mal graft density reflects the dendrimer graft density on the surface.
Figure 2.
Schematic representation of ELISA plate modification with 2.0 kDa and 3.4 kDa PEGs using EDC and HOBt in pH 6.5 MES buffer; and immobilization of PDP-functionalized G4-OH (G4-OH-PDP, 3) and EMCH on the PEGylated ELISA plate. Immobilization reaction on the ELISA plate was carried out at 4°C under nitrogen in PBS buffer with EDTA.
The dendrimer immobilization was carried out under nitrogen atmosphere at 4°C for 3 h followed by addition of 2-thioethanol to react with unreacted maleimide groups. The dendrimer modified plate was washed, dried, and stored at −20°C for several months with no signs of reduced reactivity, suggesting that the plate is stable and have an appreciable life time.
3.3 Immobilization of Antibody on Dendrimer Modified ELISA Plate
Orientation of the antibody plays a vital role in increasing the sensitivity and specificity of the biosensing platform. Sensitivity of an immunosensor can be increased by controlling the orientation of the antibody on the sensor surface. This in fact is the key step towards lowering the detection limit, as improper immobilization of the antibody leads to reduction of its binding with the analyte. Binding activity of the antibody also decreases when it binds to a solid surface of the ELISA plate.52 The reduction in activity of the antibody is due to a combination of several factors such as steric hindrance, denaturation of protein and random orientation.53 Antibodies are glycoproteins with 3-12% carbohydrate chains and most N-glycosylation sites are located in constant region (Fc) of the heavy chains. Glycosylation of these sites has little effect on binding activity of the antibodies.54 The available antibody immobilization methods include covalent coupling of the amino groups of lysine residue and using an intermediate protein that binds to the Fc region of the antibody.55,56 These methods often reduce antigen-antibody binding activity due to direct chemical modification of antigen binding site.57 In order to overcome such limitations, we have designed a biosensing platform which provides an appropriate orientation of the antibody and thereby lowers the detection limit of the biomarker. We modified the hydroxyl groups of carbohydrate in constant region which has the least effect on the antigen binding site. We converted the hydroxyl group to aldehyde group on the antibody, and reacted with the hydrazine groups of the dendrimer to get an amine linkage between dendrimer and antibody.
Mouse anti-human TNF-α antibodies were immobilized in the constant region of the oligosaccharide moieties and reacted with dendrimer modified ELISA plate having hydrazide surface groups.44 The carbohydrate vicinal hydroxyl groups in the constant region of the antibody were oxidized to aldehyde groups by sodium periodate (NaIO4) in PBS buffer (pH 7.0) (Figure 3). The number of oxidized sites generated by NaIO4 depends on its pH, temperature, reaction time and concentration of the oxidizing agent.58 Mild oxidation conditions were chosen to preserve the immunoreactivity of the antibody. The antibody (MAB610, 50 μg) in PBS (pH 6.8) was oxidized by 0.1 mg NaIO4 (0.47 mM) for 15 min at 4°C in the dark. We established the degree of antibody oxidation by Purpald test with formaldehyde as a calibrator, suggesting that approximately 8 aldehyde groups per antibody were present59 (Figure S-3).
Figure 3.
Schematic representation of antibody immobilization on the ELISA plate modified with multifunctional dendrimer (3).
The aldehyde groups are essential for reaction with hydrazide end groups of the dendrimer to form a hydrazone linkage. After oxidation, the antibody was quickly separated from the oxidizing reagent by Amicon® centrifugal filter tube, and gel filtration with concomitant buffer exchange. The purified oxidized antibody was dissolved in PBS buffer (pH 8.0) and allowed to react with dendrimer-functionalized ELISA plate at 4°C for 3 h to get hydrazone Schiff base. To overcome the instability of the Schiff base intermediate, a stable secondary amine linkage between antibody and dendrimer was established in situ by reductive amination with sodium cyanoborohydride in PBS buffer (pH 8.0).60 The reaction was completed by adding ethanolamine to block the unreacted aldehyde groups.
3.4 ELISA Evaluation of Dendrimer-modified Surface
The performance of the dendrimer-modified ELISA plate was evaluated by assaying the recombinant human TNF-α with the sandwich-type ELISA protocol using biotinylated goat anti-human TNF-α as detection antibody, streptavidin-HRP as reporter and tetramethylbenzidine (TMB) as substrate. Various known concentrations of TNF-α in the range of 0-300 pg mL−1 were assayed and a typical dose-response curve was acquired. We maintained the reaction time (2 h) for immobilization of the antibody on the sensor surface to be the same in the dendrimer-modified and in the kit plate (standard assay for TNF-α). The standard curve for TNF-α alone and TNF-α in the mixture of cytokines are shown in Figure 4. These data were compared with commercially available TNF-α ELISA kit, and a plate coated with mouse anti-human TNF-α capture antibodies for physical adsorption (Table 1).
Figure 4.
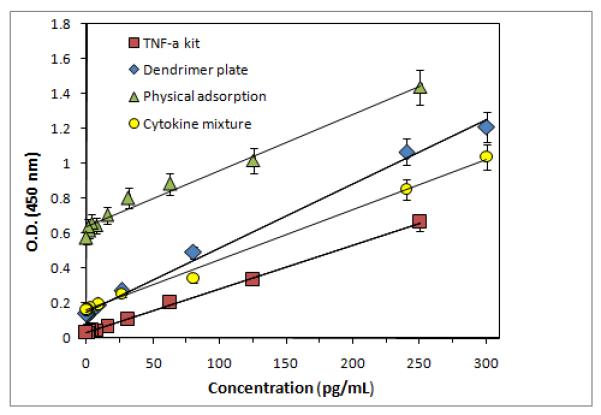
Standard curves for detection of TNF-α concentration using dendrimer plate, TNF-α kit plate, and the plate prepared by physical adsorption.
Table 1.
Detection of TNF-α concentrations in dendrimer-modified plate, plate prepared by physical adsorption and commercial TNF-α kit using ELISA (TMB detection) method.
| TNF-α Conc. (pg mL−1) |
Dendrimer Plate (O.D.) |
TNF-α Conc. (Cytok. mix.) (pg mL−1) |
Dendrimer Plate (O.D.) |
Dendrimer Plate Luminol (R.L.U.) |
TNF-α Conc. (pg mL−1) |
Physical Adsorption (O.D.) |
TNF-α Kit (O.D.) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 300 | 1.213 | 300 | 1.048 | 13414.6 | |||
| 240 | 1.067 | 240 | 0.800 | 10879.5 | 250 | 1.440 | 0.6625 |
| 80 | 0.493 | 80 | 0.423 | 4496.5 | 125 | 1.018 | 0.3335 |
| 26.6 | 0.270 | 26.6 | 0.262 | 1939.9 | 62.5 | 0.884 | 0.2045 |
| 8.88 | 0.192 | 8.88 | 0.188 | 1304.8 | 31.2 | 0.804 | 0.1070 |
| 2.96 | 0.151 | 2.96 | 0.171 | 1063.9 | 15.6 | 0.705 | 0.0655 |
| 0.98 | 0.137 | 0.98 | 0.164 | 876.8 | 7.5 | 0.646 | 0.0470 |
| 0.32 | 0.143 | 0.32 | 0.169 | 985.5 | 3.75 | 0.662 | 0.0420 |
| 0.11 | 0.141 | 0.11 | 0.158 | 975.5 | 1.87 | 0.618 | 0.0350 |
| 0.037 | 0.136 | 0.037 | 0.160 | 923.6 | 0.94 | 0.640 | 0.0335 |
| 0 | 0.138 | 0 | 0.164 | 834.1 | 0 | 0.576 | 0.0315 |
| R2 | 0.996 | 0.995 | 0.995 | 0.980 | 0.9980 | ||
|
| |||||||
| LLDa | 0.15 | 0.17 | 943.0 | 0.68 | 0.0340 | ||
|
| |||||||
| Sensitivity (pg mL−1) |
<0.48 | <1.14 | <0.39 | <8.0 | <1.280 | ||
The lower limit of detection (LLD) of TNF-α was determined by adding two STDs to the mean OD of three zero standard replicates and calculating the corresponding concentration from the standard curve.
The linear regression equation of the dendrimer plate for TNF-α was A = 0.1502 + 0.0037*C[TNF-α] with a linear regression coefficient, R = 0.996. The lower limit of detection (LLD) of TNF-α was determined by adding two standards to the mean OD of three zero standards replicates, and calculating the corresponding concentration from the standard curve. The dendrimer-modified plate, the LLD was found to be 0.48 pg mL−1 by TMB detection method (Table 1). The detection methods for TNF-α such as radioimmunoassay, bioassays, chemiluminescence, immuno-PCR assay and fluorescence immunoassay have limitation due to higher background signal associated with non-specific protein adsorption. The dendrimer-based improved capture assay in the present study has a detection limit superior to that of other previously reported TNF-α assay, except that obtained by Hun et al. where a fluoroimmunoassay which further enhances the detection component of the method used.23 The dendrimer modified plate showed a ~three-fold improvement in the detection limit compared to the kit plate (0.48 vs. 1.28 pg mL−1). This finding suggests that conjugation of the antibody to the dendrimer preserved its activity and dendrimer-based assay performed better than the commercially available ELISA kit. The results also suggest that the modification of the antibody in the Fc region did not affect the binding of the antigen and at the same time provides a favorable orientation on the plate surface. The presence of dendrimer on the plate might have played a role in improving the sensitivity by increasing the number of antibody attached to it and thereby increasing the number of antigen binding to it. The dendrimer plate showed significant improvement (~17-fold) in detection limit compared to physical adsorption (0.48 vs. 8.00 pg mL−1). When the concentration of TNF-α is zero, we found background absorbance 4-5 times more than the kit. This is due to the fact that we are modifying the ELISA plate with PEGs and dendrimer, which would result in some non-specific UV absorption. The commercial kit is proprietary, and presumably involves many ingredients to reduce this UV signal. Despite this, the sensitivity of the dendrimer-based ELISA plate for TNF-α is much higher (3-fold) than for the commercial kit.
The sensitivity was obtained from standard curve, as a slope of the regression line of the signal vs concentration plot (Figure 4). The slope of the dose-response curve for the dendrimer plate was steeper compared to the standard kit plate (0.0037 vs 0.0025), which can be explained by the enhanced binding efficiency of the antibodies immobilized on the dendrimer plate. From the standard curve, an enhanced signal was obtained at lower concentration in dendrimer-based plate. We also performed a chemiluminescence assay for both dendrimer plate and ELISA kit for TNF-α, and found that the sensitivity was similar to colorimetric assay (Table 1). This is in agreement with literature data that the chemiluminescence method may not give improved sensitivity for every cytokine.61 The overall specificity of the dendrimer-based immunosensors toward TNF-α was studied by preparing a mixture of cytokines. The standard solution containing 300 pg mL−1 of each of the following: TNF-α, IL-1α, and IL-6 were used to prepare dilutions in the range of 0-300 pg mL−1 and the selectivity of the dendrimer modified ELISA plate was examined. We did not find any significant difference in O.D. compared to the result obtained in the presence of TNF-α alone, but there is a two-fold decrease in sensitivity 2-fold (Figure 4).
The precision and reproducibility of the dendrimer-based assay was estimated by calculating the relative standard deviation (RSD) for three zero-standard replicates and coefficient of variation (CV) for both intra-assay and inter-assay variability respectively (Table-2). For immunoassays such as ELISA, intra- or inter-assay variability should be lower than 8−15% to be considered precise enough for its use.62 The dendrimer plate had good precision with RSD of 5.4 %. CV for intra-assay variability was calculated between OD values of triplicates within each run of two independent assay runs, for each dilution concentration, and it ranged from 0.09 to 13.0%. The inter-assay variability was determined by calculating the CV using OD values obtained from two triplicates of independent assay runs, and it ranged between 2.08 and 13.39%. This indicates that the assays on dendrimer modified plates are reproducible.
Table 2.
Precision profile of intra- and inter-assay %CV of dendrimer plate for TNF-α detection.
| Intra-assay | Inter-assay | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | TNF-α Conc. (pg ml−1) |
SD | CV(%) | N | TNF-α Conc. (pg ml−1) |
SD | CV(%) |
|
| |||||||
| 2 | 300 | 0.096 | 8.74 | 4 | 300 | 0.157 | 0.157 |
| 2 | 240 | 0.038 | 3.28 | 4 | 240 | 0.177 | 0.177 |
| 2 | 80 | 0.0007 | 0.13 | 4 | 80 | 0.013 | 0.013 |
| 3 | 26.6 | 0.015 | 5.6 | 4 | 26.6 | 0.0408 | 0.0408 |
| 3 | 8.88 | 0.017 | 8.9 | 6 | 8.88 | 0.0198 | 0.0198 |
| 3 | 2.96 | 0.016 | 10.3 | 5 | 2.96 | 0.0158 | 0.0158 |
| 3 | 0.98 | 0.009 | 6.9 | 6 | 0.98 | 0.0104 | 0.0104 |
| 3 | 0.32 | 0.010 | 7.0 | 5 | 0.32 | 0.0096 | 0.0096 |
| 3 | 0.11 | 0.018 | 13.0 | 4 | 0.11 | 0.0114 | 0.0114 |
| 3 | 0.037 | 0.002 | 1.3 | 5 | 0.037 | 0.0049 | 0.0049 |
| 3 | 0 | 0.007 | 5.4 | 5 | 0 | 0.006 | 0.006 |
N: Number of samples; SD: Standard deviation; and CV: Coefficient of variation.
4. Conclusions
TNF-α is an important biomarker for various inflammatory diseases, including chorioaminionitis. The ability to measure very low concentrations of TNF-α accurately and precisely is very important for the early diagnosis of infection/inflammation in the amniotic fluid. In the present study, a dendrimer-based biosensor platform was developed for the detection of TNF-α cytokine. Thiol-functionalized PAMAM G4-OH dendrimers were covalently immobilized onto a maleimide-terminated PEG-modified ELISA plate. The dendrimer-modified ELISA plate was reacted to oxidized capture antibody by reacting hydrazide groups of dendrimer and aldehyde groups of the antibody. This allowed the capture antibody to have favorable orientation of the antigen binding sites toward the analyte phase. The dendrimer-modified ELISA plate showed a detection limit of 0.48 pg mL−1 for TMB detection, which was significantly better than the commercial ELISA kit for TNF-α. The increased sensitivity of the modified plate can also be due to the dendrimer-templated presentation of the capture-antibody, which ultimately increases the binding of the number of TNF-α on the plate. The dendrimer based diagnostic nanodevice proved to have improved sensitivity and specificity and can be used for the detection of a wide range of cytokines and biomarkers.
Supplementary Material
Acknowledgment
This research was supported by the Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH/DHHS), and the Ralph C. Wilson Foundation for Biomedical Research, and WSU nanotechnology effort.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication.As a service to our customers we are providing this early version of the manuscript.The manuscript will undergo copyediting, typesetting, and review of the resulting proofbefore it is published in its final citable form. Please note that during the productionprocess errors may be discovered which could affect the content, and all legal disclaimersthat apply to the journal pertain.
Supporting Information The proton NMR, MALDI TOF mass spectra and HPLC chromatogram of PDP-functionalized G4-OH (G4-OH-PDP, 3) and NMR spectrum of model compound 5 are included in supporting information.
References
- [1].Gotsch F, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, Espinoza J, Hassan SS. Clin. Obstet. Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- [2].Rüetschi U, Rosén A, Karlsson G, Zetterberg H, Rymo L, Hagberg H, Jacobsson B. J. Proteome Res. 2005;4:2236–2242. doi: 10.1021/pr050139e. [DOI] [PubMed] [Google Scholar]
- [3].Slattery MM, Morrison JJ. The Lancet. 2002;360:1489–1497. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- [4].Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Seminar L. Fetal and Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McIntire DD, Leveno KJ. Obstet. Gynecol. 2008;111:35–41. doi: 10.1097/01.AOG.0000297311.33046.73. [DOI] [PubMed] [Google Scholar]
- [6].Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Am. J. Obstet. Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- [7].Harirah H, Donia SE, Hsu C-D. Obstet. Gynecol. 2002;99:80–84. doi: 10.1016/s0029-7844(01)01632-5. [DOI] [PubMed] [Google Scholar]
- [8].Romero R, Mazor M. Clin. Obstet. Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- [9].Romero R, Chaiworapongsa T, Espinoza JJ. The J. Nutrition. 2003;133:1688S–1973S. doi: 10.1093/jn/133.5.1668S. [DOI] [PubMed] [Google Scholar]
- [10].Edward T, Gross H, Weinberger A, Cohen H. Med Gen Med. 2006;8:25. [Google Scholar]
- [11].Clarke DJ, Branton RL. Exp. Neurol. 2002;176:154–162. doi: 10.1006/exnr.2002.7911. [DOI] [PubMed] [Google Scholar]
- [12].Titelbaum DS, Degenhardt A, Kinkel RP. Am. J. Neuroradiol. 2005;26:1548–1550. [PMC free article] [PubMed] [Google Scholar]
- [13].Sakao S, Tatsumi K, Igari H, Shino Y, Shirasawa H, Kuriyama T. Am. J. Respir. Crit. Care Med. 2001;163:420–422. doi: 10.1164/ajrccm.163.2.2006031. [DOI] [PubMed] [Google Scholar]
- [14].von Haehling S, Hopkinson NS, Polkey MI, Niethammer M, Anker SD, Genth-Zotz S. Wien. Klin. Wochenschr. 2009;121:303–308. doi: 10.1007/s00508-009-1186-7. [DOI] [PubMed] [Google Scholar]
- [15].Yoon B, Jun J, Romero R, Park K, Gomez R, Choi J, Kim I. Am. J. Obstet. Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- [16].Teppo AM, Maury CP. Clin. Chem. 1987;33:2024–2027. [PubMed] [Google Scholar]
- [17].Jones LJ, Singer VL. Anal. Biochem. 2001;293:8–15. doi: 10.1006/abio.2001.5116. [DOI] [PubMed] [Google Scholar]
- [18].Berthier F, Lambert C, Genin C, Bienvenu J. Clin. Chem. Lab. Med. 1999;37:593–599. doi: 10.1515/CCLM.1999.092. [DOI] [PubMed] [Google Scholar]
- [19].Luo L, Zhang Z, Ma L. Anal. Chim. Acta. 2005;539:277–282. [Google Scholar]
- [20].Hurst GB, Buchanan MV, Foote LJ, Kennel SJ. Anal. Chem. 1999;71:4727–4733. doi: 10.1021/ac9905423. [DOI] [PubMed] [Google Scholar]
- [21].Saito K, Kobayashi D, Komatsu M, Yajima T, Yagihashi A, Ishikawa Y, Minami R, Watanabe N. Clin. Chem. 2000;46:1703–1704. [PubMed] [Google Scholar]
- [22].Saito K, Kobayashi D, Sasaki M, Araake H, Kida T, Yagihashi A, Yajima T, Kameshima H, Watanabe N. Clin. Chem. 1999;45:665–669. [PubMed] [Google Scholar]
- [23].Hun X, Zhang Z. Talanta. 2007;73:366–371. doi: 10.1016/j.talanta.2007.03.059. [DOI] [PubMed] [Google Scholar]
- [24].Wang J, Liu G, Engelhard MH, Lin Y. Anal. Chem. 2006;78:6974–6979. doi: 10.1021/ac060809f. [DOI] [PubMed] [Google Scholar]
- [25].Liu Z, Amiridis MD. J. Phys. Chem. B. 2005;109:16866–16872. doi: 10.1021/jp0535240. [DOI] [PubMed] [Google Scholar]
- [26].Ajikumar PK, Ng JK, Tang YC, Lee JY, Stephanopoulos G, Too H-P. Langmuir. 2007;23:5670–5677. doi: 10.1021/la063717u. [DOI] [PubMed] [Google Scholar]
- [27].Menjoge AR, Kannan RM, Tomalia DA. Drug Discov. Today. 2010;15:171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- [28].Frechet JMJ, Tomalia DA. Dendrimers and Other Dendritic Polymers. Wiley; Chichester, New York: 2001. [Google Scholar]
- [29].Bosman AW, Janssen HM, Meijer EW. Chem. Rev. 1999;99:1665–1688. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]
- [30].Yoon HC, Hong M-Y, Kim H-S. Anal. Biochem. 2000;282:121–128. doi: 10.1006/abio.2000.4608. [DOI] [PubMed] [Google Scholar]
- [31].Sayed-Sweet Y, Hedstrand DM, Spinder R, Tomalia DA. J. Mater. Chem. 1997;7:1199–1205. [Google Scholar]
- [32].Benters R, Niemeyer CM, Drutschmann D, Blohm D, Wöhrle D. Nucleic Acids Res. 2002;30:e10. doi: 10.1093/nar/30.2.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Benters R, Niemeyer CM, Wöhrle D. Chem Bio chem. 2001;2:686–694. doi: 10.1002/1439-7633(20010903)2:9<686::AID-CBIC686>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- [34].Degenhart GH, Dordi B, Schönherr H, Vancso GJ. Langmuir. 2004;20:6216–6224. doi: 10.1021/la049580u. [DOI] [PubMed] [Google Scholar]
- [35].Das J, Aziz MA, Yang H. J. Am. Chem. Soc. 2006;128:16022–16023. doi: 10.1021/ja0672167. [DOI] [PubMed] [Google Scholar]
- [36].Selvaraju T, Das J, Han SW. Biosensors Bioelectronics. 2008;23:932–938. doi: 10.1016/j.bios.2007.09.010. [DOI] [PubMed] [Google Scholar]
- [37].Zhu Y, Zhu H, Yang X, Xu L, Li C. Electroanalysis. 2007;19:698–703. [Google Scholar]
- [38].Bustos EB, Jiménez Ma. G.G., Díaz-Sánchez BR, Juaristi E, Chapmam TW, Godínez LA. Talanta. 2007;72:1586–1592. doi: 10.1016/j.talanta.2007.02.017. [DOI] [PubMed] [Google Scholar]
- [39].Yoon HC, Hong M-Y, Kim H-S. Langmuir. 2001;17:1234–1239. [Google Scholar]
- [40].Mark SS, Sandhyarani N, Zhu C, Campagnolo C, Batt CA. Langmuir. 2004;20:6808–6817. doi: 10.1021/la0495276. [DOI] [PubMed] [Google Scholar]
- [41].Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK. Acc. Chem. Res. 2001;34:181–190. doi: 10.1021/ar000110a. [DOI] [PubMed] [Google Scholar]
- [42].Gröhn F, Bauer BJ, Akpalu YA, Jackson CL, Amis EJ. Macromolecules. 2000;13:6042–6050. [Google Scholar]
- [43].Esumi K, Akiyama S, Yoshimura T. Langmuir. 2003;19:7679–7681. [Google Scholar]
- [44].Han HJ, Kannan RM, Wang S, Mao G, Pedro-Kusanovic J, Romero R. Adv. Funct. Mater. 2010;20:409–421. doi: 10.1002/adfm.200901293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rebeski DE, Winger EM, Shin Y-K, Lelenta M, Robinson MM, Varecka R, Crowther JR. J. Immunol. Methods. 1999;226:85–92. doi: 10.1016/s0022-1759(99)00051-4. [DOI] [PubMed] [Google Scholar]
- [46].Bosnjakovic A, Mishra MK, Ren W, Kurtoglu YE, Shi T, Fan D, Kannan RM. Nanomedicine: Nanotechnology, Biology and Medicine. 2010;7:284–94. doi: 10.1016/j.nano.2010.10.008. [DOI] [PubMed] [Google Scholar]
- [47].Menjoge AR, Navath RS, Asad A, Kannan S, Kim CJ, Romero R, Kannan RM. Biomaterials. 2010;31:5007–5021. doi: 10.1016/j.biomaterials.2010.02.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Getz EB, Xiao M, Chakrabarty T, Cook R, Selvin PR. Anal. Biochem. 1999;273:73–80. doi: 10.1006/abio.1999.4203. [DOI] [PubMed] [Google Scholar]
- [49].Navath RS, Kurtoglu YE, Wang B, Kannan S, Romero R, Kannan RM. Bioconjug. Chem. 2008;19:2446–2455. doi: 10.1021/bc800342d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nagasaki Y, Kobayashi H, Katsuyama Y, Jomura T, Sakura T. J. Colloid Interface Sci. 2007;309:524–530. doi: 10.1016/j.jcis.2006.12.079. [DOI] [PubMed] [Google Scholar]
- [51].Nie T, Baldwin AD, Yamaguchi N, Kiick KL. J. Control Release. 2007;122:287–296. doi: 10.1016/j.jconrel.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Choi J-W, Chun BS, Oh B-K, Lee W, Lee WH. Colloids Surf. B: Biointerfaces. 2005;40:173–177. doi: 10.1016/j.colsurfb.2004.10.020. [DOI] [PubMed] [Google Scholar]
- [53].Jung Y, Kang HJ, Lee JM, Jung SO, Yun WS, Chung SJ, Chung BH. Anal. Biochem. 2008;374:99–105. doi: 10.1016/j.ab.2007.10.022. [DOI] [PubMed] [Google Scholar]
- [54].Donadel G, Calabro A, Sigounas G, Hascall VC, Notkins AL, Harindranath N. Glycobiology. 1994;4:491–496. doi: 10.1093/glycob/4.4.491. [DOI] [PubMed] [Google Scholar]
- [55].Snopok B, Yurchenko M, Szekely L, Klein G, Kashuba E. Anal. Bioanal. Chem. 2006;386:2063–2073. doi: 10.1007/s00216-006-0867-6. [DOI] [PubMed] [Google Scholar]
- [56].Fowler JM, Stuart MC, Wong DKY. Anal. Chem. 2007;79:350–354. doi: 10.1021/ac061175f. [DOI] [PubMed] [Google Scholar]
- [57].Jung Y, Jeong JY, Chung BH. Analyst. 2008;133:697–701. doi: 10.1039/b800014j. [DOI] [PubMed] [Google Scholar]
- [58].Abraham R, Moller D, Gabel D, Senter P, Hellström I, Hellström KE. J. Immunol. Methods. 1991;144:77–86. doi: 10.1016/0022-1759(91)90233-6. [DOI] [PubMed] [Google Scholar]
- [59].Jacobsen NW, Dickinson RG. Anal. Chem. 1974;46:298–299. [Google Scholar]
- [60].Roberts JC, Adams YE, Tomalia DA, Mercer-Smith JA, Lavallee DK. Bioconjug. Chem. 1990;1:305–308. doi: 10.1021/bc00005a001. [DOI] [PubMed] [Google Scholar]
- [61].Samineni S, Parvataneni S, Kelly C, Gangur V, Karmaus W, Brooks K. J. Immunoass. Immunochem. 2006;27:183–193. doi: 10.1080/15321810600573051. [DOI] [PubMed] [Google Scholar]
- [62].Laczka O, Baldrich E, del Campo FJ, Munoz FX. Anal. Bioanal. Chem. 2008;391:2825–2835. doi: 10.1007/s00216-008-2199-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.