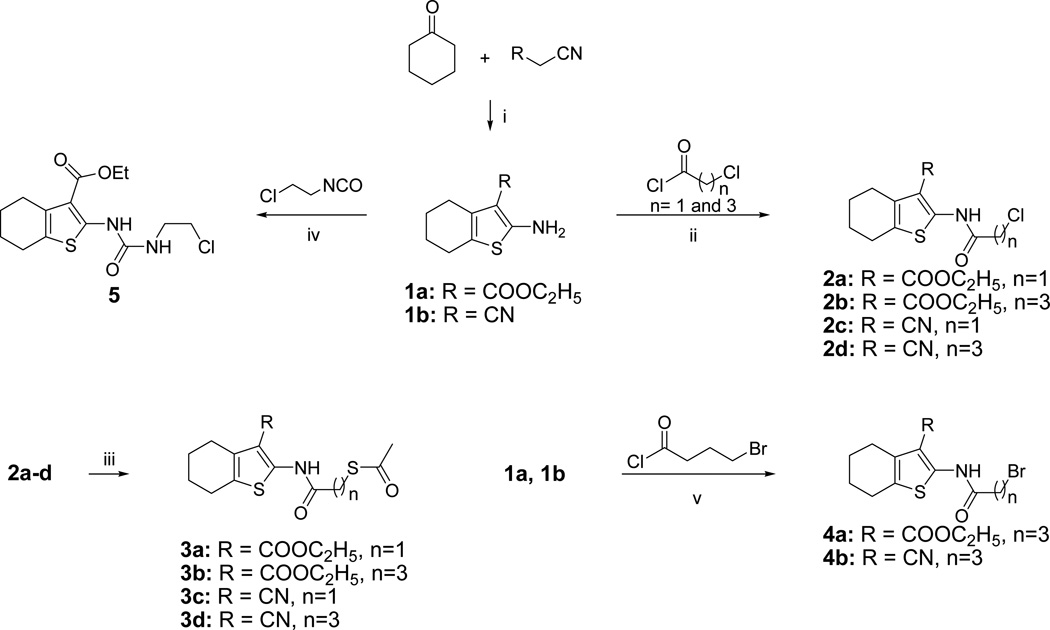
Scheme 1.
Synthesis of Tetrahydrobenzo[b]thiophenesa
aReagent and conditions: (i): sulfur, N-ethylmorpholine, EtOH, 1a (27%), 1b (33%); (ii): Et3N, CH2Cl2, rt, 2a (11%), 2b (9%), 2c (15%), 2d (29%); (iii): KSCOCH3, DMF, rt, 3a (62%), 3b (66%), 3c (96%), 3d (78%); (iv) toluene, 112 °C, 3 h (53%); (v): CH2Cl2, rt, 4a (39%), 4b (67%).