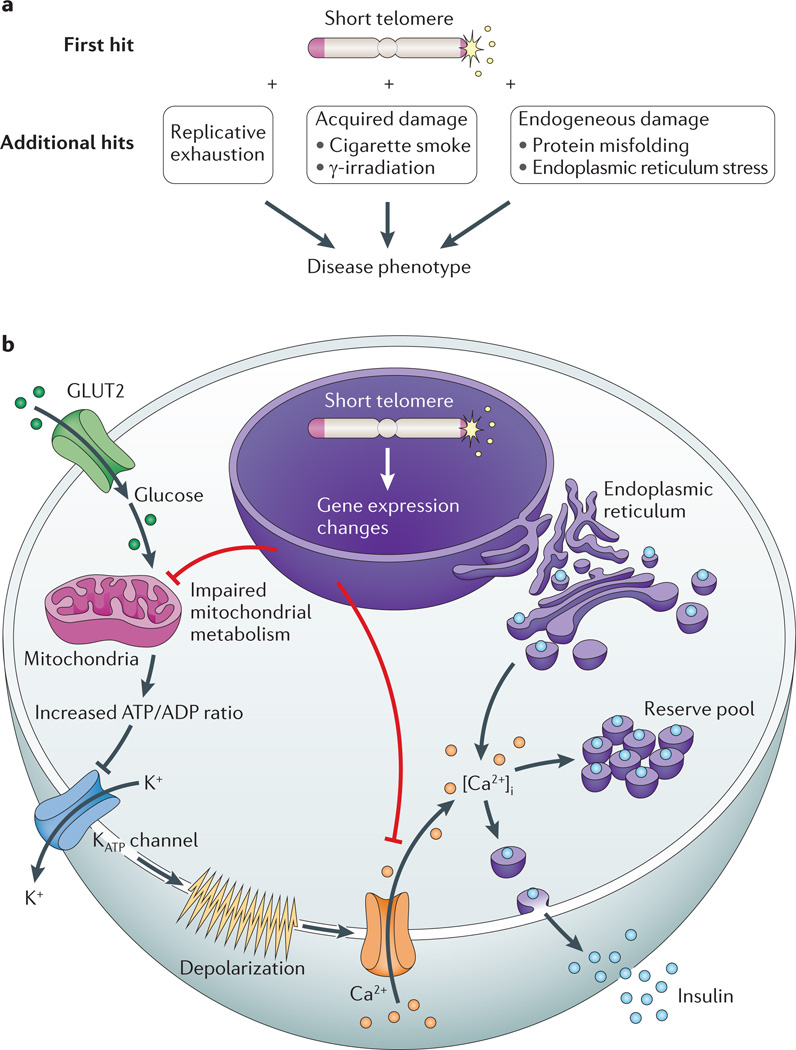
Figure 5. Mechanisms of telomere-mediated disease in slow-turnover tissues.
a | Telomere length lowers the threshold to endogenous and exogenous damage, which is hypothesized to precipitate disease in slow-turnover tissues. b | A schematic of an insulin-producing β-cell showing the mechanism of a telomere-mediated insulin exocytosis defect. Glucose passively enters β-cells through the glucose transporter type 2 (GLUT2; also known as SLC2A2). Following glycolysis, ATP is generated by oxidative phosphorylation in the mitochondria. The net cytosolic change of the ATP/ADP ratio leads to closure of ATP-dependent K+ channels and opening of Ca2+ channels. The influx of extracellular Ca2+ results in an increase in the concentration of free, intracellular Ca2+ ([Ca2+]i), which triggers the release of insulin from both reserve and back-up pools. Short telomeres cause gene expression changes in pancreatic islets, which are associated with global metabolic dysregulation, mitochondrial dysfunction and concurrent defects in glucose-dependent and glucose-independent Ca2+ handling.