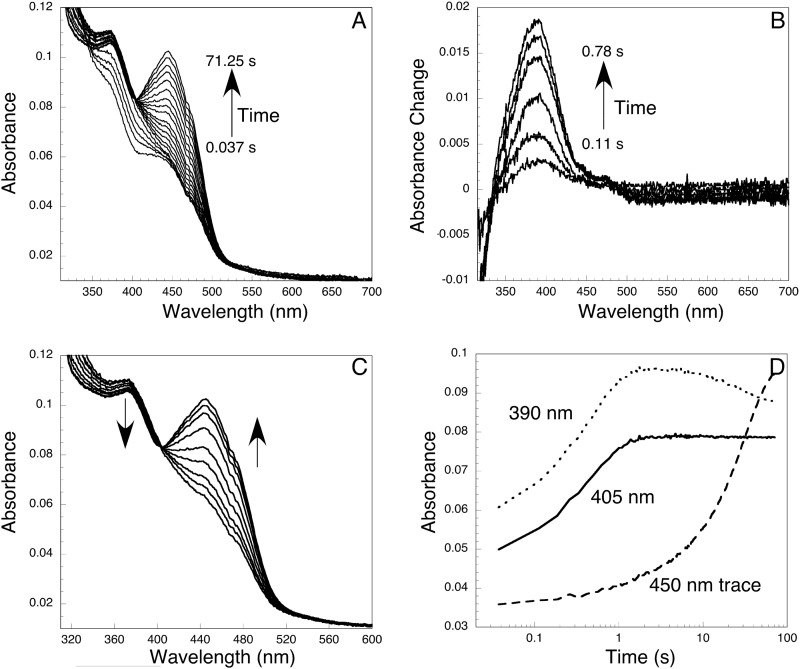
FIGURE 4.
Formation and decomposition of the C4a-(hydro)peroxyflavin intermediate. Equal volumes of reduced YUC6 (18 μm) and oxygen-saturated buffer were mixed in the stopped-flow instrument. A, spectra recorded after mixing reduced YUC6 with oxygen. The first spectrum was taken at 0.037 s, and the last one was taken at 71.25 s. B, formation of the C4a-(hydro)peroxyflavin as shown by an increase of absorbance at 381 nm. These difference spectra, which clarify the appearance of the C4a-(hydro)peroxyflavin, were calculated by subtracting the initial spectrum of reduced YUC6 from each successive spectrum out to 0.78 s. Within the first second, decomposition of the intermediate was minimal. No absorbance change at 450 nm was observed within this period. C, decomposition of C4a-(hydro)peroxyflavin to FAD. Approximately 1 s after mixing, the intermediate started to decompose. Spectra shown were recorded in the time window of 2–4 s. D, kinetics of the formation and decomposition of the C4a-(hydro)peroxyflavin. The formation of the intermediate was evident in the traces recorded at 390 nm and 405 nm. The decomposition was followed at 450 nm. The isosbestic point between C4a-intermediate and oxidized FAD was at 405 nm.