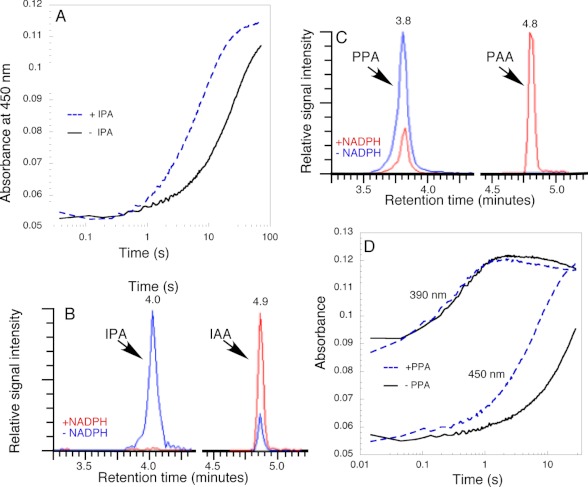
FIGURE 5.
Reaction of C4a-(hydro)peroxyflavin with IPA and PPA. A, comparison of the conversion of C4a-intermediate to oxidized FAD with and without 250 μm IPA. Other conditions are the same as in Fig. 4A. B, HPLC analysis of the products generated by the reaction of YUC6 with IPA. IPA is converted to IAA in the presence of NADPH. Note that there was a small amount nonenzymatic conversion of IPA to IAA in the absence of NADPH. C, YUC6 catalyzing the conversion of PPA to PAA. Note that the reaction is cleaner than that with IPA, and no nonenzymatic conversion of PPA to PAA is observed. D, comparison of the formation and decomposition of the C4a-intermediate with and without the substrate PPA (250 μm). Other conditions are the same as in Fig. 4A. The formation of the C4a-intermediate was followed at the first part of the 390-nm traces. Note that the substrate PPA did not affect the formation of the C4a-intermediate. The decomposition of the C4a-intermediate was followed at 450 nm. Note that PPA substantially accelerated the rate of conversion of the C4a-intermediate to FAD.