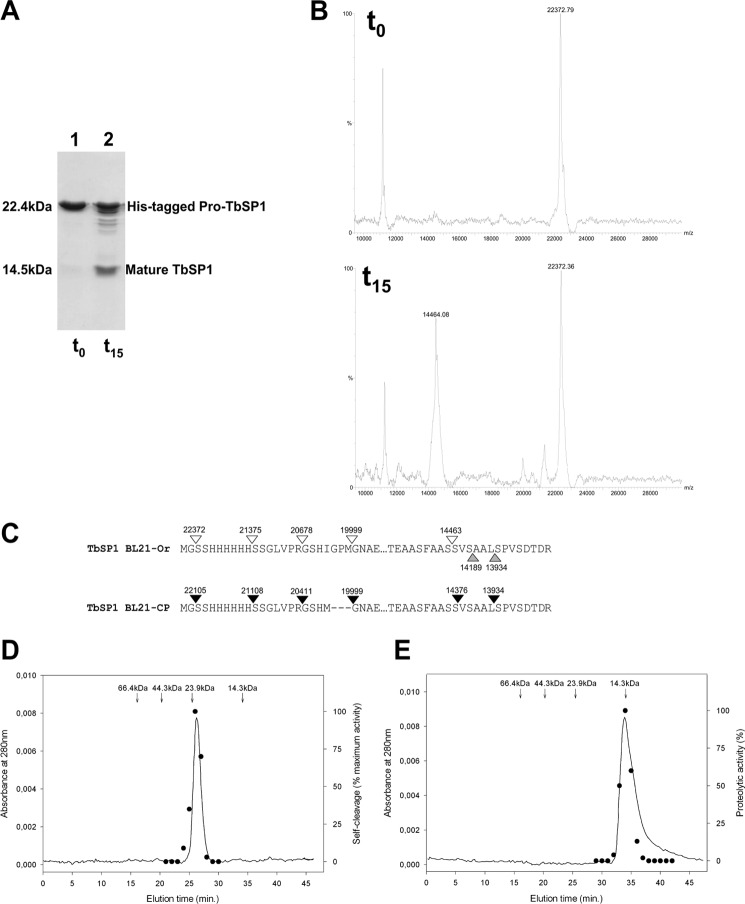
FIGURE 4.
Proteolytic self-processing of TbSP1. A, SDS-PAGE profile of recombinant 22-kDa TbSP1 purified from the BL21-Ori strain (lane 1, t0 control) and of the same protein incubated at 37 °C for 15 h at a concentration of 20 μm (lane 2, t15). B, MALDI-TOF analysis of the t0 (upper spectrum) and the t15 (lower spectrum) protein samples utilized for the experiment in A (see “Experimental Procedures” for details). C, map of the cleavage sites identified by MALDI-TOF analysis of the digestion products generated upon incubation of TbSP1 from the BL21-Ori (empty arrowheads) or the BL21-CP (filled arrowheads) strain; upward pointing gray arrowheads indicate the N-terminal amino acid residues revealed by x-ray analysis of TbSP1 crystals. D, recombinant 22-kDa TbSP1, prepurified by metal affinity chromatography, was run on a Superdex 75 5/150 GL column at 4 °C (solid line), and the autoproteolytic activity of individual fractions was assayed by SDS-PAGE analysis as in A (dots); the data shown are from one of three replicates that produced nearly identical results. The elution times and molecular weights of protein standards run on the same column are reported above the chromatogram. E, same as D with the mature (14.5-kDa) form of TbSP1. Individual peak fractions were assayed for proteolytic activity (black dots, right axis) using proteolytically inactive TbSP1 from inclusion bodies as substrate (see “Experimental Procedures” for details).