FIGURE 5.
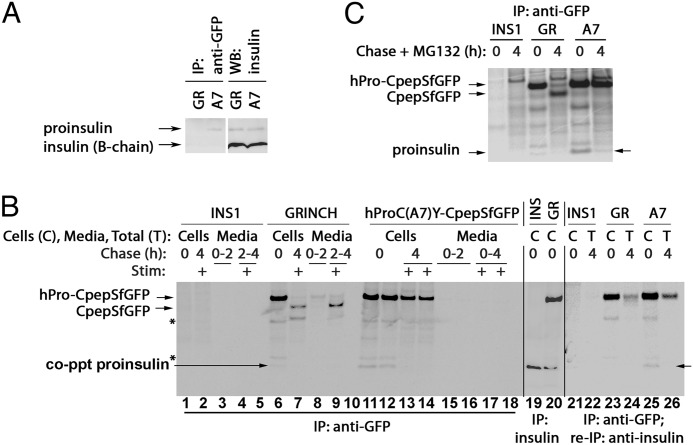
Wild-type and mutant hPro-CpepSfGFP coprecipitate endogenous proinsulin. A, GRINCH (GR) and hProC(A7)Y-CpepSfGFP (A7) cells were either lysed and resolved by reducing SDS-PAGE in 4–12% acrylamide gradient gels and electrotransfer to nitrocellulose for direct immunoblotting (WB) with anti-insulin (second set of lanes) or immunoprecipitated (IP) with anti-GFP antibodies before proceeding with the same immunoblotting analysis (first set of lanes). Note that hProC(A7)Y-CpepSfGFP cells do not have increased intracellular proinsulin, yet anti-GFP immunoprecipitation selectively coprecipitates endogenous proinsulin from hProC(A7)Y-CpepSfGFP cells. B, parental INS1 (INS), GRINCH, and hProC(A7)Y-CpepSfGFP cells were labeled with 35S-Met/Cys for 30 min, chased for the first 2 h under unstimulated (low glucose) conditions, and then stimulated with secretagogue (Stim: +) for another 2 h. The media were collected, and the cells were lysed at the indicated chase times. The samples were immunoprecipitated with anti-GFP antibodies (lanes 1–18, lanes 5 and 10 are blanks), resolved using reducing SDS-PAGE in 4–12% acrylamide gradient gels, and analyzed by phosphorimaging. As a control for the positions of hPro-CpepSfGFP and proinsulin bands, INS1 and GRINCH cells were labeled with 35S-Met/Cys for 1 h and immunoprecipitated with anti-insulin (lanes 19 and 20). Note that after stimulation, processed CpepSfGFP was robustly secreted from GRINCH cells (lane 9). In lanes 21-26, cells were labeled and chased, and samples were collected as before, but the 4-h media and cell lysates were combined into a single sample to generate total (T). Anti-GFP immunoprecipitates samples were then reboiled in SDS, diluted into complete immunoprecipitation buffer, reimmunoprecipitated with anti-insulin, resolved using SDS-PAGE in 4–12% acrylamide gradient gels, and analyzed by phosphorimaging. Note that coprecipitation (co-ppt) of endogenous proinsulin became undetectable after a 4-h chase (lanes 7, 13, 14, and 26). The identities of the two bands marked with asterisks are unknown. C, parental INS1, GRINCH, and hProC(A7)Y-CpepSfGFP cells were labeled with 35S-Met/Cys as in B but either lysed immediately or chased for 4 h in the presence of 10 μm MG132 before immunoprecipitation with anti-GFP and analysis as in B. Loss of proinsulin coprecipitation with hProC(A7)Y-SfCpepGFP was at least in part related to ERAD because some endogenous proinsulin could still be coprecipitated at 4 h in cells treated with MG132 (arrow at right). In contrast, loss of the weak endogenous proinsulin coprecipitation with hPro-CpepSfGFP in GRINCH cells corresponds to processing to insulin and CpepSfGFP, consistent with Fig. 2A.