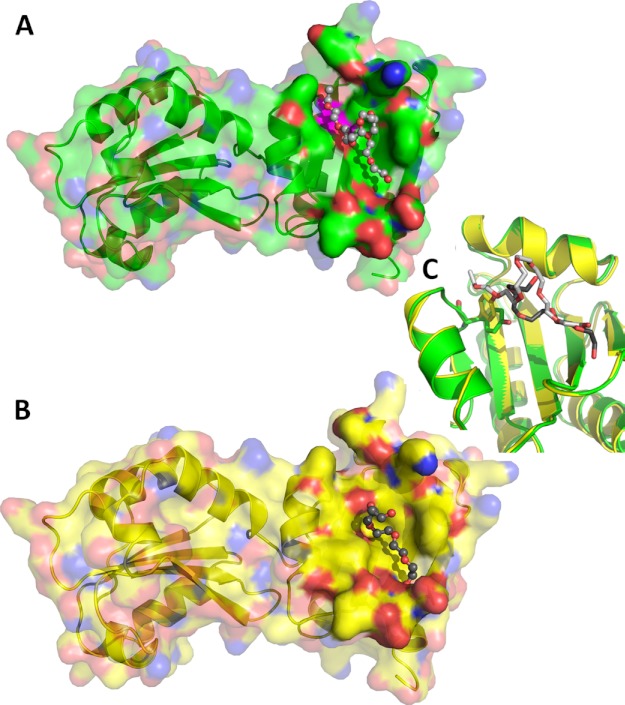
FIGURE 3.
Structural features of ERp27. A and B, surface representation of the ERp27 A chain (A) and D chain (B) with their respective bound PEG molecule. The protein is shown in ribbon representation with a semitransparent surface with oxygen atoms in red, nitrogen atoms in blue, and carbon atoms in green (A chain) and yellow (D chain), respectively. The surface is rendered solid near the bound PEG molecules, which are represented as ball and stick models with red oxygen atoms and carbon atoms in light gray (A chain) and dark gray (D chain), respectively. Tyr-147 of the A chain has been mapped onto the surface in magenta. C, close-up view of the superimposed A (green) and D chains (yellow) illustrating the rotation of the Tyr-147 side chain. Tyr-147 (stick model) rotates its hydroxyl group away from the surface, extending the hydrophobic cleft from 9.5 Å to over 14 Å in response to the interaction with the PEG molecules.