Abstract
OBJECTIVES
Atrial fibrillation is the most common cardiac arrhythmia and is associated with significant morbidity and mortality. The classic cut-and-sew maze procedure is successful in 85–95% of patients. However, the technical complexity has prompted modifications of the maze procedure. The objective of this study was to retrospectively evaluate the clinical safety and efficacy of the maze treatment performed at our institution.
METHODS
From March 2001 until February 2009, 169 patients underwent a modified maze procedure for atrial fibrillation at the Erasmus MC, Rotterdam. Patient characteristics, surgical procedure and follow-up data were obtained by reviewing the medical charts and consulting with the referring physicians. The efficacy of the procedure as measured by AF recurrence was analysed with a repeated measurements model. The quality of life of the patients was assessed with the SF-36 (a short-form health survey with 36 questions) questionnaire and compared with that of the general Dutch population.
RESULTS
Of the 169 patients who underwent a modified maze procedure, 163 had their maze procedure as a concomitant procedure. The 30-day mortality rate was 4.7% (n = 8). The rate of post-procedural AF recurrence varied significantly over time (P < 0.0001). Decreased left ventricular function, increased age and higher preoperative creatinine levels were predictors of AF recurrence. Quality of life, as measured with the SF-36 questionnaire, was comparable with that of the Dutch population for all health domains.
CONCLUSIONS
Concomitant maze is a relatively safe treatment that eliminates atrial fibrillation in the majority of patients, although the probability of recurrent AF increases with the passage of time. Decreased left ventricular function, increased age and higher preoperative creatinine levels are associated with an increased risk of AF recurrence.
Keywords: Atrial fibrillation, Maze procedure, Arrhythmia therapy, Quality of life
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia. AF has a prevalence of 0.1–2.3% in people older than 40 years, 5.9% in those older than 65 years and 10.5% in those aged 85 and above [1]. With the ageing of the general population, AF represents a growing health problem.
AF is associated with increased mortality and morbidity, and the irregular heartbeat sometimes causes patient discomfort and anxiety [2]. Approximately 16% of all thrombo-embolic strokes are associated with AF [3]. Stroke remains the most catastrophic consequence of AF.
The cut-and-sew maze procedure is able to cure AF in 85–95% of patients [4, 5]. However, the technical complexity of the classic maze has prompted modifications of the procedure. A variety of patterns and devices with different energy sources have been introduced to perform a maze-like procedure more rapidly and safely [5]. However, the safety and efficacy of the different maze procedures vary between studies [6, 7].
The objective of this study was to evaluate our single-centre experience with the clinical application of modified maze procedures. In particular, the data analysed in this article address issues related to the probability and the predictors of the recurrence of AF, as well as the factors related to early survival following the maze procedure. Furthermore, the quality of life following the maze procedure was measured, and a statistical comparison was made with the Dutch population.
MATERIALS AND METHODS
Subjects
Between March 2001 and February 2009, 169 patients underwent a maze procedure as a surgical treatment for arrhythmia related to acquired heart disease in the Department of Cardiothoracic Surgery of Erasmus MC, Rotterdam. The patients were invited to participate in the study after providing written informed consent. Some patients did not respond to the quality-of-life questionnaire and some did not consent to the collection of follow-up data. Therefore, the safety analysis is based on 169 patients and the efficacy analysis on 162 patients. Ninety-nine patients participated in the quality-of-life assessment and consented to the registration of their data and its use in this publication.
The Medical Ethical Committee of the Erasmus MC approved this study (MEC 2009-231). The study was conducted in accordance with the criteria of Good Clinical Practice (Declaration of Helsinki, 2004; www.ICH.org).
Data collection
Retrospectively, we obtained the demographic and medical characteristics, the preoperative AF classification (an interventional classification of either paroxysmal or chronic [8]), the EuroSCORE [9], the operation characteristics, cardiovascular risk factors, cardiac rhythm, use of antiarrhythmic drugs and complications after surgery through a (electronic) medical chart review and consultation with the referring physicians.
The following major post-procedural complications were scored: haemodynamic instability (requiring an intra-aortic balloon pump), temporary renal failure (continuous veno-venous haemofiltration), respiratory failure (reintubation), re-exploration for persistent blood loss, reoperation cases during the same admission, sternal wound infection (requiring re-intervention), myocardial infarction (conform European Society of Cardiology guidelines), stroke (without full recovery) and a postoperative need for a permanent pacemaker.
The cardiac rhythm at follow-up was determined by the referring cardiologist based on electrocardiography (ECG) or 24-h Holter monitoring, and the rhythm was scored as a sinus rhythm (SR), AF/atrial flutter or a pacemaker rhythm. Our maze procedures were considered successful in the case of restoration of SR.
The quality of life in the survivors was cross-sectionally assessed between August 2009 and November 2010 using the SF-36 questionnaire [10]. The SF-36 is a short-form health survey with 36 questions. It is a commonly used and validated quality-of-life questionnaire. It yields an 8-scale profile of functional health and well-being scores. The patient scores were compared with those of the general Dutch population [11].
Surgical procedure
Standard cardiopulmonary bypass was established through median sternotomy, performed mostly via bicaval cannulation, and cardiac arrest was obtained under aortic cross-clamping and repeated with antegrade and/or retrograde cold crystalloid cardioplegia. Of the 169 surgical ablation procedures, 163 were performed concomitantly with valve surgery, coronary artery bypass grafting (CABG) or both.
During the study period, the maze procedures were performed using a variety of patterns and devices with different energy sources, including radiofrequency (RF), cryothermy (CRT) and high-intensity focused ultrasound (HIFU) mainly based on the surgeon's discretion and actual market developments.
The ablation lines created were as follows: most of the incisions currently used in the Cox maze III procedure were replaced with RF or CRT lines, and the standard left atrial incision in Waterston's groove and an incision in the right atrium were performed when a bi-atrial maze was done by cut-and-sew. These incisions were used to enter the atrial cavities. During this period, some studies have shown that only left atrial ablation has results similar to those of bi-atrial ablation [6, 12]. For this reason, during the course of this study, right atrial ablation was no longer an integral part of the maze procedure. The HIFU device was developed to only ablate the left atrium, and it was used on the beating heart.
Postoperative treatment of atrial fibrillation
AF in the first few postoperative days was treated through the correction of electrolyte disturbances, the correction of the intravascular fluid status and the exclusion of significant pericardial effusion. Pharmacological treatment with a Class II antiarrhythmic drug (metoprolol), Class III antiarrhythmic drugs (amiodarone/sotalol) or digoxin was started based on the patient characteristics, the clinical presentation and the prior pharmacological treatment of the patient. If these interventions did not result in SR, an electrocardioversion was performed. The referring cardiologists for patients discharged with AF were advised to consider an electrocardioversion approximately 6 weeks after the procedure.
Statistical analysis
Continuous data are presented as the mean ± standard deviation if normally distributed and as the median ± inter-quartile range (IQR) if skewed (Kolmogorov–Smirnov test). Categorical data are presented as numbers and proportions. Differences between the demographic and surgical characteristics of the patients with SR and AF were evaluated by the χ2 test for categorical data and by the Student's t-test for continuous data if normally distributed. The Mann–Whitney U-test was used if the continuous data were skewed. To test the different energy sources, a one-way analysis of variance was used.
The efficacy of the surgical procedure as measured by AF recurrence was assessed with a repeated-measurement analysis using generalized mixed effects models [13]. We relaxed the common linearity assumption for the effect of time on the probability of AF using natural cubic splines with two internal knots (placed at the corresponding percentiles of the observed follow-up times) in both the fixed-effects and the random-effects parts of the model (i.e. time is allowed to possibly have a non-linear association with the probability for AF) [14]. In addition, we also controlled for differences in the probability of AF at baseline for age, gender, the type of AF, the energy source and a history of mitral valve surgery. The models were fitted using the adaptive Gauss–Hermite rule with four quadrature points [13].
The results of the SF-36 questionnaire were compared with the population norms by the Wilcoxon rank-sum test with Bonferroni correction. This latter correction indicates that the allowable significance level for each SF-36 subscale was P < 0.00625 (0.05/8 subscales).
The data were registered in a dedicated database. The statistical analysis was conducted using SPSS 17 and R version 2.13.1 (8 July 2011) with the package lme4. The significance level was set to 5%.
RESULTS
Preoperative data
From March 2001 until February 2009, 169 patients who underwent a modified maze procedure were included. The patient characteristics are outlined in Table 1.
Table 1:
Patient characteristics
| Total (n = 169) | |
|---|---|
| Age (mean [SD] years) | 63.7 (11.0) |
| Male (n (%)) | 95 (56.2) |
| Type of AF | |
| Paroxysmal (n (%)) | 60 (35.5) |
| Long-standing (n (%)) | 109 (64.5) |
| Diabetes mellitus (n (%)) | 22 (13.1) |
| Hypertension (n (%)) | 51 (30.4) |
| Hyperlipidaemia (n (%)) | 25 (14.9) |
| BMI (mean (SD)) | 26.5 (4.0) |
| Pulmonary hypertension (n (%)) | 8 (4.7) |
| Previous cardiac surgery (n (%)) | 12 (7.1) |
| Preoperative creatinine (mean (SD)) | 90.9 (22.37) |
| Left ventricular functioning moderate/poor (n (%)) | 12 (7.1) |
| EuroSCORE (mean (SD)) | 4.73 (1.9) |
| Logistic EuroSCORE (mean (SD)) | 4.18 (2.8) |
| NYHA III–IV (n (%)) | 70 (41.4) |
BMI: body mass index; EuroSCORE: European System for Cardiac Operative Risk Evaluation; NYHA: New York Heart Association for dyspnoea.
Operative data
Table 2 displays the operation characteristics. Of the 169 modified maze procedures, 96 were performed with RF ablation, 20 ablations were conducted with CRT and 52 patients were treated with HIFU. In one lone AF patient, a classic Cox maze was performed. RF ablation was mainly performed before 2007, and HIFU was performed from 2007 onwards. Bi-atrial ablation was performed in 88 patients with RF ablations and in 7 patients with CRT ablation.
Table 2:
Operation characteristics
| Total (n = 169) | |
|---|---|
| Type of maze | |
| Cut-and-sew (n (%)) | 1 (0.6) |
| RF bi-atrial ablation (n (%)) | 88 (52.1) |
| RF left atrium (n (%)) | 8 (4.7) |
| CRT left atrium (n (%)) | 13 (7.7) |
| CRT bi-atrial (n (%)) | 7 (4.1) |
| HIFU (n (%)) | 52 (30.6) |
| Concomitant proceduresa | |
| None | 6 |
| Mitral valve repair | 89 |
| Mitral valve replacement | 38 |
| Aortic valve replacement | 30 |
| Aortic valve repair | 1 |
| Tricuspid valve repair | 46 |
| CABG | 39 |
| Other cardiac surgery | 11 |
| ECC time | |
| Mean (SD), min | 183.1 (76.4) |
| Median (IQR) | 169.0 (81.0) |
| AOX time (mean (SD), min) | 119.7 (52.0) |
| Bi-auricular amputation (n (%)) | 18 (10.7) |
| Left auricular amputation (n (%)) | 113 (66.9) |
AOX: aortic cross-clamp time; CRT: cryothermic ablation; ECC: extracorporeal circulation; HIFU: high-intensity focused ultrasound; RF: radiofrequency ablation.
aOverlapping categories.
Early mortality and morbidity
Early mortality (<30 days) occurred in 8 (4.7%) patients. Four of the early deaths occurred during surgery (Table 3). The other 4 cases of early postoperative mortality were caused by massive cerebral infarction in 1 patient and multiple organ failure in 3 other patients.
Table 3:
Intraoperative mortality
| Age | Gender | EuroSCORE (%) | Procedure | Cause of death |
|---|---|---|---|---|
| 68 | Male | 12.0 | AVR, MVP, CRT | Cardiac failure |
| 75 | Female | 5.8 | AVR, MVR, RF | Exsanguination due to mitral annular rupture |
| 78 | Female | 7.0 | MVR, RF | Exsanguination due to mitral annular rupture |
| 78 | Male | 13.9 | AVR, LV aneurysmectomy, CABG, HIFU | Cardiac failure |
AVR: aortic valve replacement; CABG: coronary artery bypass grafting; CRT: cryothermic maze; HIFU: high-intensity focused ultrasound maze; LV: left ventricular; MVP: mitral valve annuloplasty; MVR: mitral valve replacement, RF: radiofrequency.
There was one device-related complication; this maze procedure was aborted due to bleeding while fitting the HIFU device. Overall complications were seen in 30 patients (18.2%) who experienced one or more complications, and these are outlined in Table 4.
Table 4:
Complications <30 days
| Total (n = 169) | |
|---|---|
| Haemodynamic instability/cardiac failure | 9 (8) |
| Reintubation for respiratory failure | 9 (3) |
| Renal failure | 7 (3) |
| Stroke | 2 (1) |
| Myocardial infarction | 1 (1) |
| Sternal wound infection | 5 (0) |
| Re-do cases | 3 (1) |
| Re-exploration for persistent blood loss | 14 (0) |
| Need for a permanent pacemaker | 4 (0) |
Number of patients with complications. A patient can have more than one complication.
n: Number of cases with <30-day mortality.
Late survival
During a median follow-up of 45.6 months (IQR 37.5), another 20 patients died. The cause of mortality during the follow-up was cardiac in 9 patients, non-cardiac in 6 and unknown in 5. Of the 9 patients with cardiac death, 1 died of multiorgan failure after cardiac arrest, 2 of cardiogenic shock, 3 of cardiac arrhythmia and 1 of heart failure. There was 1 case of aortic rupture and 1 of aortic valve prosthetic endocarditis. Of the 6 patients who died of non-cardiac causes, 2 died of pneumonia, 1 of sepsis, 1 of lung cancer and 1 of intracerebral bleeding. Finally, in 1 patient, treatment was stopped for a non-cardiac reason. The cumulative survival rate was 81.9% (95% confidence interval, CI: 78.6–85.2) at 6.5 years.
Postoperative atrial fibrillation
The repeated-measurement analysis is based on 162 patients with a total of 1934 postoperative rhythm registrations. Of the 162 patients, there were 131 who had one or more abnormal rhythm registrations. Holter monitoring was performed for 24 h in 44 patients with SR on ECG who had complaints of palpitations. This revealed periods of AF/atrial flutter in 21 patients. Postoperatively, 23 patients (14%) did not show SR in a rhythm registrations made. The total number of registrations for different periods of time postoperatively is shown in Fig. 1. Postoperatively, 76 patients (48%) were discharged with SR. During follow-up, 39% of the patients were registered as having AF at 6 months after the procedure, 47% had AF at 1 year and 46% had AF at 2 years. Figure 2 depicts the fitted probability for AF over time in a virtual patient, showing that the probability of AF after the procedure varies with time, remaining quite low in the first two postoperative years and increasing gradually thereafter. The test for non-linearity revealed that the patient-specific profiles for the log odds of failure to restore SR after the procedure are indeed non-linear in time (likelihood ratio test = 289.9, df = 9, P-value <0.0001) [14]. The significant effect of time suggests that the patient-specific profiles for the probability of AF were not constant in time. Table 5 presents the estimated relative risks under the full model. We observe that decreased left ventricular functioning, time and age are the most important predictors.
Figure 1:
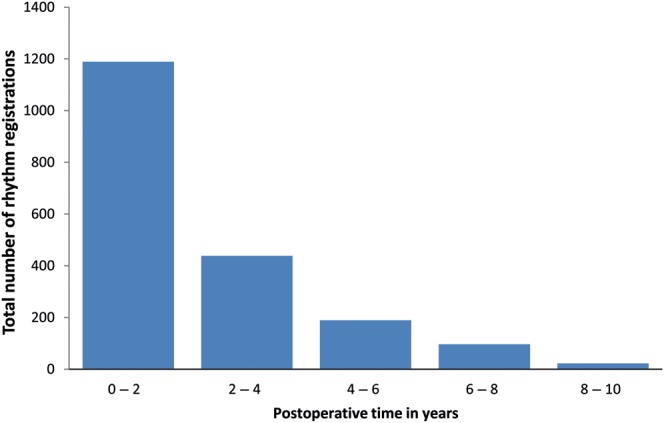
Total number of rhythm registrations over different periods of postoperative time in years.
Figure 2:
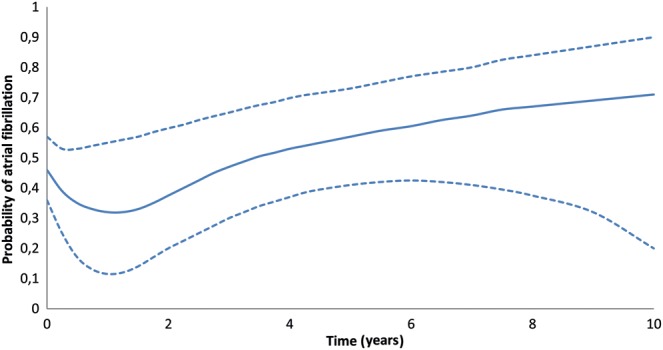
Probability of AF over time (years) after surgery. Adjusted to age 62, mitral valve repair, male, long-standing AF, radiofrequency ablation, without diabetes mellitus and hyperlipidaemia, preoperative creatinine 91 mmol/l, good left ventricular function and bi-atrial ablation. The dashed lines denote 95% pointwise confidence intervals.
Table 5:
Estimated predictors of the recurrence of AF after the maze procedure
| Relative risk (95% CI) | P-value | |
|---|---|---|
| Age | 1.031 (1.008–1.056) | 0.0070 |
| Male | 1.017 (0.639–1.619) | 0.9413 |
| Diabetes | 1.132 (0.568–2.257) | 0.7254 |
| Hypertension | 0.728 (0.428–1.239) | 0.2409 |
| Hyperlipidaemia | 0.546 (0.277–1.077) | 0.0804 |
| Increased preoperative creatinine | 1.012 (1.000–1.024) | 0.0471 |
| Chronic AF | 1.563 (0.975–2.508) | 0.0637 |
| LV function moderate/poor | 3.554 (1.530–8.255) | 0.0032 |
| Left atrial ablation only | 1.868 (0.791–4.408) | 0.1643 |
| Mitral valve replacement | 0.819 (0.400–1.678) | 0.5840 |
| Mitral valve repair | 0.710 (0.393– 1.283) | 0.2558 |
| CRT ablation | 0.537 (0.218–1.324) | 0.1775 |
| HIFU ablation | 0.984 (0.377–2.566) | 0.9743 |
Estimated coefficients of the recurrence of AF under the full model.
AF: atrial fibrillation; CRT: cryothermic; HIFU: high-intensity focused ultrasound; LV: left ventricular.
Quality of life
The quality of life was assessed in 99 patients, on average 41.1 ± 26 months (median 45.6, IQR 37.5) after surgery. The patients were divided into four age categories: age 16–40 years (n = 6), 40–60 years (n = 15), 60–70 years (n = 34) and age >70 (n = 44). The quality of life was divided into eight subscales: physical function, role physical, bodily pain, general health, vitality, social function, role emotional and mental health. In none of the age categories and subscales, was there a significant difference between the patients who underwent the maze procedure and the general population.
DISCUSSION
Our study showed that the probability of AF after the maze procedure varies with time, remaining quite low during the first postoperative years and increasing gradually thereafter. Decreased left ventricular function, age and increased preoperative creatinine levels were associated with a decreased probability of sustained SR. While the 30-day mortality in our study population was relatively high, it can be explained by preoperative characteristics and by the complexity of the cardiac procedures.
The probability of sustained SR depends on the time of measurement. During the immediate postoperative period, many patients experience a period of arrhythmia. Often, these arrhythmias terminate after a few weeks due to scar formation and atrial remodelling after the procedure. Early arrhythmias might be prevented by continuous bi-atrial pacing and could result in shortened hospital stays and decreased hospital costs [15]. The effects of continuous pacing on late recurrence of AF, however, are still unknown.
After several years, the probability of AF appears to increase, which is consistent with previous research [16]. This can be explained by continuous dilatation of the atria after the procedure. Through this mechanism, the ablation lines can become too far apart and macro-re-entry can develop. However, the maze procedure reduces the atrial size, particularly in patients whose mitral valve function was restored during surgery [17].
Another explanation for late AF recurrence is the on-going deterioration of atrial tissue associated with ageing and the possibility of atrial remodelling at the time of the maze procedure. The various forms of adverse atrial remodelling are known to cause AF: anatomical, electrical, molecular and histological, resulting in macro-re-entry, which is the substrate for AF. These alterations or remodelling can, for example, be caused by congestive heart failure and arrhythmias and might be not fully reversible [18].
Older age is a risk factor for AF. We confirm the findings from previous research that it is also a risk factor for the recurrence of AF after the procedure [19]. Age might also be a marker for the duration of the AF preoperatively, which has been previously associated with an increased risk of failure [16]. In addition, repeated episodes of AF result in chronic atrial stress, which leads to atrial fibroses [20]. Furthermore, atrial interstitial fibrosis has been observed with ageing and might create the substrate for AF [21]. This might be a reason for the reduced possibility for reverse remodelling and, therefore, an increase in AF after the procedure in older patients.
In our experience, higher preoperative creatinine levels lower the probability of sustained SR. Reduced kidney functioning is a known risk factor for AF [22]. This is expected because patients with kidney dysfunction are more likely to have hypertension and diabetes mellitus, which are known risk factors for AF. However, these factors were not predictive in our model. An upregulated renin–angiotensin–aldosterone system has also been associated with the increased occurrence of atrial interstitial fibrosis. Therefore, an angio converting enzyme (ACE) inhibitor could help to prevent interstitial fibrosis and thereby the recurrence of AF. Furthermore, ACE inhibitors are known to reduce left atrial size and new onset AF [23].
Our results show that the procedure might be less effective in patients with decreased left ventricular function, and many surgeons are reluctant to perform this procedure in patients with a low ejection fraction because of possibly increased risks. However, previous research has shown that the procedure in well-selected patients can be safely performed [24]. In addition, patients with low ejection fraction, in particular, could benefit from the improved ejection fraction after the restoration of SR or reduction of the AF burden, which may contribute to improved long-term outcomes, including mortality.
The maze procedure is often performed late in the course of AF and/or valvular disease, as shown in our experience. The duration of AF is probably not only an indicator of the degree of atrial tissue disease, but also reflects the decreased chances of the restoration of SR [18]. In patients with long-standing AF who have undergone the maze procedure, the late recurrence of AF is expected because of the significant amount of diseased atrial tissue, which is more susceptible to arrhythmia, and the procedure fails to lead to a total reversal of the remodelling process. Therefore, the late recurrence of AF might be reduced by earlier surgical intervention.
The rate of early mortality in our study population was relatively high. This can be explained by the preoperative characteristics and by the complexity of the cardiac procedures. The estimated early mortality risk calculated with the EuroSCORE was comparable with our results. A previous study [16] also showed an in-hospital mortality rate similar to our results. It is unlikely that this mortality is related to the maze procedure. Moreover, in combined procedures, the modified maze procedures are a relatively simple adjunct to the procedure.
Health-related quality of life is severely impaired among patients with AF, which has been demonstrated in several previous studies. Because AF is often a concomitant issue in a complex cardiac disease, it is not easy to attribute (loss of) quality of life directly to AF. In this study, we found that patients who have undergone a maze procedure have a health status similar to that of the general Dutch population. As we did not have a baseline measurement for the quality of life, we cannot show an improvement in the quality of life, if any, as has been shown in previous studies [25].
The current study has several limitations that should be considered. This is a small retrospective cohort, single-centre study and, therefore, it has shortcomings such as incomplete data, changes in the ablation modalities and differences in the lesion sets over time. Likewise, the heterogeneity of the background diseases in our population could also have influenced the results of the reoccurrence of AF. Subgroup analyses of the different lesion sets, ablation devices and the type of AF were, because of the small numbers, not meaningful. Furthermore, the outcomes of procedures for the ablation of AF are influenced by the thoroughness of follow-up and the method of assessment of cardiac rhythm. The electrocardiogram is a ‘snap-shot’ in time and has limited ability to detect those patients who may have transient atrial arrhythmias in the follow-up period. A better method would be 1 or 7 days of Holter monitoring, but for routine follow-up, this is not widely used. The strength of our study is the repeated-measurement analysis. In this way, we tried to solve the ‘snap-shot’ effect. The quality-of-life assessment was, of course, only completed by the survivors. Further validation of the SF-36 questionnaire in postoperative concomitant maze patients has to be confirmed.
In conclusion, in our clinical experience, the modified maze procedure is a relatively safe treatment, and in combined procedures, it is a relatively simple adjunct to the operation. The quality of life after the procedure is comparable with that of the general Dutch population. The efficacy of our maze procedure varied over time, but it eliminated AF in the majority of patients. Decreased left ventricular function, older age and higher preoperative creatinine levels were associated with an increased risk of the recurrence of AF. Early surgical intervention may increase the success rate of the restoration of SR after the modified maze procedure.
Conflict of interest: none declared.
REFERENCES
- 1.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–73. [PubMed] [Google Scholar]
- 2.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell L, Skanes A. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention and treatment of atrial fibrillation following cardiac surgery. Can J Cardiol. 2011;27:91–7. doi: 10.1016/j.cjca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Cox JL, Schuessler RB, Boineau JP. The development of the Maze procedure for the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2000;12:2–14. doi: 10.1016/s1043-0679(00)70010-4. [DOI] [PubMed] [Google Scholar]
- 5.Prasad SM, Maniar HS, Camillo CJ, Schuessler RB, Boineau JP, Sundt TM, 3rd, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–8. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 6.Deneke T, Khargi K, Grewe PH, von Dryander S, Kuschkowitz F, Lawo T, et al. Left atrial versus bi-atrial Maze operation using intraoperatively cooled-tip radiofrequency ablation in patients undergoing open-heart surgery: safety and efficacy. J Am Coll Cardiol. 2002;39:1644–50. doi: 10.1016/s0735-1097(02)01836-3. [DOI] [PubMed] [Google Scholar]
- 7.Mantovan R, Raviele A, Buja G, Bertaglia E, Cesari F, Pedrocco A, et al. Left atrial radiofrequency ablation during cardiac surgery in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:1289–95. doi: 10.1046/j.1540-8167.2003.03077.x. [DOI] [PubMed] [Google Scholar]
- 8.Cox J. The longstanding, persistent confusion surrounding surgery for atrial fibrillation. J Thorac Cardiovasc Surg. 2010;139:1374–86. doi: 10.1016/j.jtcvs.2010.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–22. doi: 10.1016/s1010-7940(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 10.Ware J, Snow K, Kosinski M, Gandek B. SF-36 health survey manual and interpretation guide. Boston. MA: New England Medical Center, the Health Institute. 1993 [Google Scholar]
- 11.Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055–68. doi: 10.1016/s0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 12.Khargi K, Hutten B, Lemke B, Deneke T. Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg. 2005;27:258–65. doi: 10.1016/j.ejcts.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Molenberghs G, Verbeke G. Models for Discrete Longitudinal Data. New York: Springer; 2005. [Google Scholar]
- 14.Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- 15.Wang W, Buehler D, Feng XD, Zhang SY. Continuous biatrial pacing to prevent early recurrence of atrial fibrillation after the Maze procedure. J Thorac Cardiovasc Surg. 2011;142:989–94. doi: 10.1016/j.jtcvs.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Beukema WP, Sie HT, Misier AR, Delnoy PP, Wellens HJ, Elvan A. Predictive factors of sustained sinus rhythm and recurrent atrial fibrillation after a radiofrequency modified Maze procedure. Eur J Cardiothorac Surg. 2008;34:771–5. doi: 10.1016/j.ejcts.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Fayad G, Le Tourneau T, Modine M, Azzaoui R, Ennezat PV, Decoene C. Endocardial radiofrequency ablation during mitral valve surgery: effect in cardiac rhythm, atrial size, and function. Ann Thorac Surg. 2005;79:1505–11. doi: 10.1016/j.athoracsur.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Thijssen VL, Ausma J, Borgers M. Structural remodelling during chronic atrial fibrillation: act of programmed cell survival. Cardiovasc Res. 2001;52:14–24. doi: 10.1016/s0008-6363(01)00367-4. [DOI] [PubMed] [Google Scholar]
- 19.Maltais S, Forcillo J, Bouchard D, Carrier M, Cartier R, Demers P, et al. Long-term results following concomitant radiofrequency modified maze ablation for atrial fibrillation. J Card Surg. 2010;25:608–13. doi: 10.1111/j.1540-8191.2010.01087.x. [DOI] [PubMed] [Google Scholar]
- 20.Schotten U, Neuberger H, Allessie MA. The role of atrial dilatation in the domestication of atrial fibrillation. Prog Biophys Mol Biol. 2003;82:151–62. doi: 10.1016/s0079-6107(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 21.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo M, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–4. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 22.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, et al. Chronic kidney disease is associated with the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:2946–53. doi: 10.1161/CIRCULATIONAHA.111.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA. 2006;296:1242–8. doi: 10.1001/jama.296.10.1242. [DOI] [PubMed] [Google Scholar]
- 24.Ad N, Henry L, Hunt S. The impact of surgical ablation in patients with low ejection fraction, heart failure, and atrial fibrillation. Eur J Cardiothorac Surg. 2011;40:70–6. doi: 10.1016/j.ejcts.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Lonnerholm S, Blomstrom P, Nilsson L, Blomstrom-Lundqvist C. A high quality of life is maintained late after Maze III surgery for atrial fibrillation. Eur J Cardiothorac Surg. 2009;36:558–62. doi: 10.1016/j.ejcts.2009.04.030. [DOI] [PubMed] [Google Scholar]


