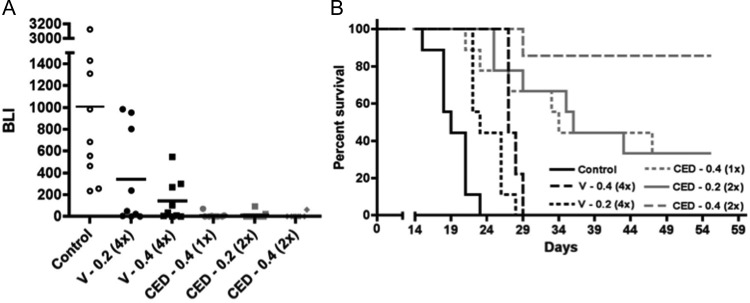
Fig. 1.
Comparison of intravascular vs CED administration of nanoliposomal irinotecan for anti-tumor activity against intracranial GBM43 and corresponding survival benefit for animal subjects. (A) Treatment group day 15 normalized bioluminescence18 (BLI) distributions (last imaging day in which all control group mice were alive). Route of administration identifiers: V, vascular administration; CED, convection enhanced delivery. Numbers following route of administration identifiers represent mg quantity of irinotecan administered with each dose; numbers in parentheses represent the number of administrations. Direct administrations were on day 5 or on days 5 and 8, and vascular administrations were on days 5, 8, 12, and 15. Student's t-test values for all 2-way comparisons are listed in Table 1s. (B) Corresponding survival plots for each treatment group. Log rank P values for all 2-way comparisons are listed in Table 2 and show that the survival benefit for mice receiving 2 CED administrations of 0.4 mg irinotecan was significantly greater (P < .05) than for any other treatment. Mice surviving at day 60, all of which had no detectable tumor by bioluminescence imaging, were euthanized, with analysis of serial H&E-stained sections of entire brains from 2 of these mice showing no detectable tumor. Control group mice in this experiment were untreated, which were established as valid for comparison by determining, in a separate experiment, that CED of vehicle caused no adverse or beneficial effect on animal survival relative to no treatment (Supplementary Figure 2). Number of mice included in the survival analysis for each treatment group (see Materials and Methods): Control = 9; CED 0.2 (2x) = 9; CED 0.4 (1x) = 9; CED 0.4 (2x) = 7; V 0.2 (4x) = 9; and V 0.4 (4x) = 9.