Fig. 2.
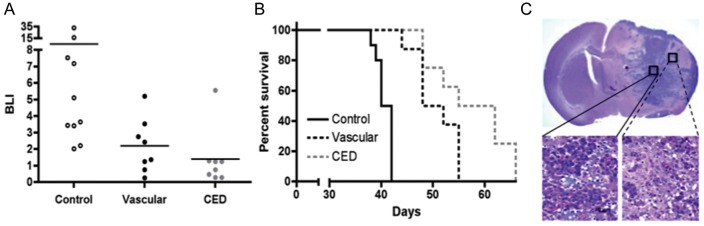
Comparison of intravascular with CED administration of nanoliposomal irinotecan for anti-tumor activity and survival benefit for mice with intracranial SF7796. (A) Treatment group day 35 bioluminescence distributions (last imaging day for which all control group mice were alive). Mice receiving vascular administration received 0.4 mg doses of irinotecan on days 20 and 24 (0.8 mg total dose), whereas mice receiving CED administration were treated just once with 0.4 mg irinotecan on day 20 after tumor cell implantation. Student's t-test values for 2-way comparisons: 0.092 for control vs vascular; 0.061 for control vs CED; and 0.359 for vascular vs CED. (B) Corresponding survival plots for each treatment group. Log rank P values for 2-way comparisons: <0.001 for control vs vascular; <0.001 for control vs CED; and 0.048 for vascular vs CED. Number of mice included in the survival analysis for each treatment group: Control = 10; Vascular = 8; CED = 8. (C) 1.25x (upper) and 40x (lower) magnifications of an H&E-stained coronal section from the brain of a control group mouse that was euthanized on day 39 because of symptoms from increasing tumor burden. Lower left panel: high cellularity at the tumor core. Lower right panel: disseminated tumor cells at the tumor periphery.
