Fig. 7.
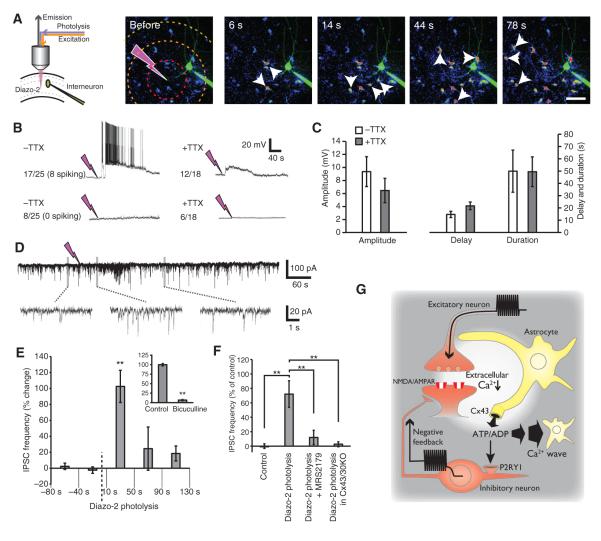
Diazo-2 photolysis evokes interneuronal depolarization and bursting. (A) Experimental setup used for photolysis of diazo-2 combined with whole-cell recordings of interneurons located ~40 to 80 μm from the site targeted by the UV beam in hippocampal slices. Time series images of the slow Ca2+ wave evoked by photolysis of diazo-2. The pipette contained Alexa Fluor 488 (green) to visualize the impaled interneuron. Small white arrows point to astrocytes. Hatched circles define the radius 50, 100, and 150 μm from the photolysis site. Scale bar, 50 μm. (B) Representative recordings of interneurons (current clamp) in response to diazo-2 photolysis without (left panel) or with TTX (1 μM, right panel). Of a total of 25 recordings, 17 interneurons exhibited a transient depolarization (membrane potential shift >2 mV), and 8 of these cells exhibited spiking activity. In the presence of TTX, 12 of a total of 18 interneurons exhibited a transient depolarization (>2 mV), whereas the remaining 6 failed to respond to diazo-2 photolysis. (C) Comparison of the amplitude, delay, and duration of diazo-2–induced depolarization in the presence and absence of TTX (n = 6 to 8 photolysis events, P > 0.05). (D) Representative traces of recordings in a CA1 pyramidal neuron (current clamp) located ~40 to 80 μm from diazo-2 photolysis site. Enlarged time scales for the indicated periods are shown below. (E) Changes in the frequency of IPSCs induced by diazo-2 photolysis plotted as a function of time before or after diazo-2 photolysis (n = 5 photolysis events, **P < 0.01). Inset: Bicuculline reduced the frequency of IPSCs during baseline conditions (20 mM n = 9 slices, **P < 0.01). (F) Comparison of the effect of photolysis of diazo-2 on IPSC frequency during control conditions (10 to 50 s), after addition of the P2Y1 receptor antagonist MRS2179 (50 μM), or in slices from mice lacking Cx43 and Cx30 (Cx43/30KO) (n = 9 slices, **P < 0.01). (G) Proposed model for [Ca2+]e as a mediator of neuron-glia signaling. Glutamatergic signaling (or MNI photolysis) triggers Ca2+ influx into neurons through AMPA- or NMDA-type glutamate, leading to a localized decrease in [Ca2+]e. Connexin hemichannels in astrocytes open in response to low [Ca2+]e, enabling the efflux of ATP. ATP triggers two events: (i) ATP activates slowly propagating Ca2+ waves in astrocytes by binding to astrocytic P2YR1, P2YR2, and P2YR4; (ii) ATP is degraded to ADP, and in turn, ADP activates interneuronal P2Y1 receptors, stimulating the depolarization and increased firing of a subpopulation of interneurons. This mode of neuron-glia signaling may act as a negative feedback mechanism to increase inhibitory transmission during excessive glutamatergic activity.