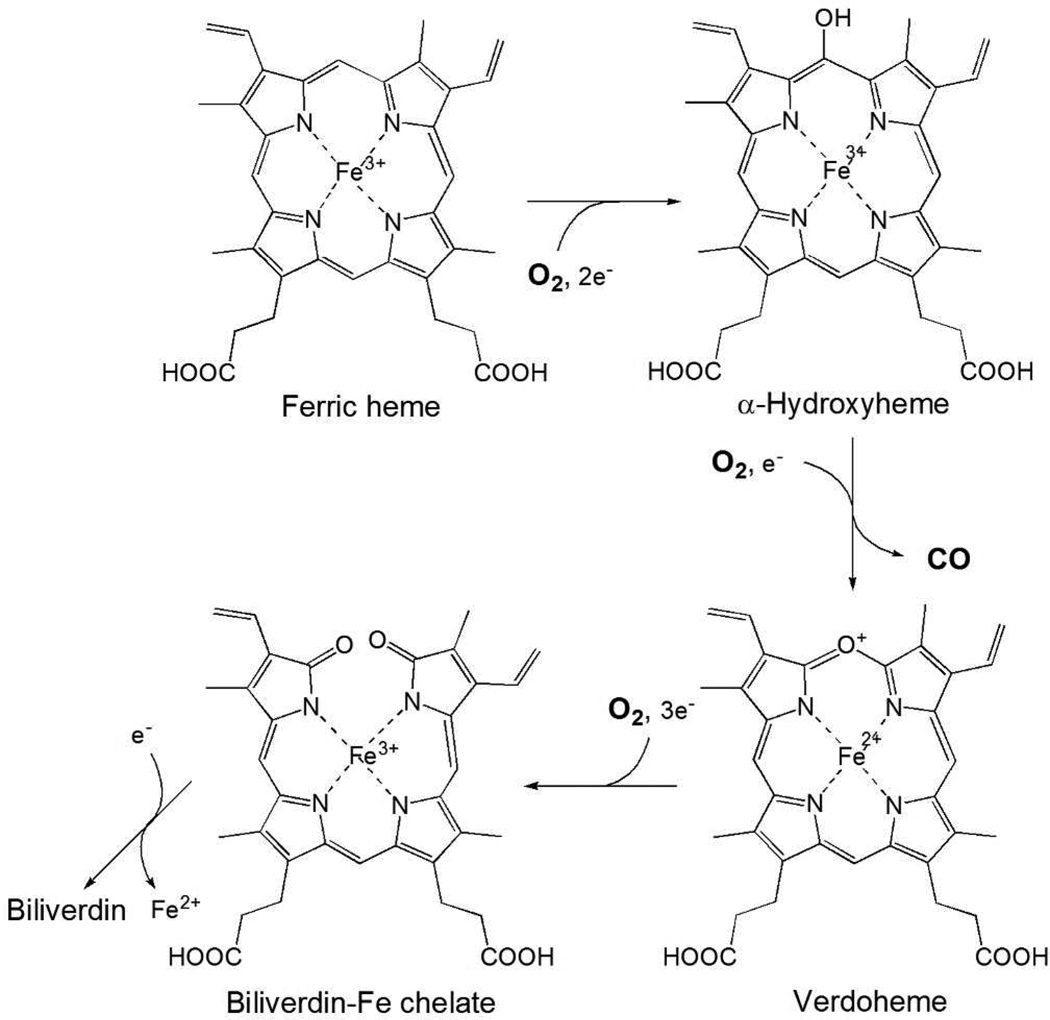
Figure 1.
Reaction scheme of HO. The overall HO reaction proceeds via a multi-step mechanism. The first step is the oxidation of heme to α-hydroxyheme, requiring O2 and reducing equivalents supplied by NADPH cytochrome P450 reductase. O2 bound to the heme iron is activated to the hydrogen peroxy species, and α-hydroxyheme is then produced. The second step is the formation of verdoheme with the concomitant release of the hydroxylated α-meso carbon as CO. The third step is the conversion of verdoheme to biliverdin-iron chelate, also requiring electrons and consuming O2. In the final step, the iron of biliverdin-iron chelate is reduced, and ferrous iron and biliverdin are released from HO. The conversion of verdoheme to ferric biliverdin-iron chelate followed by the release of biliverdin is the rate-limiting step in the HO reaction.