Figure 4. Tumor reconstitution-allografting experiment and reduced promoter CpG methylation of the STAT1 gene in PDAC cells after DAC treatment.
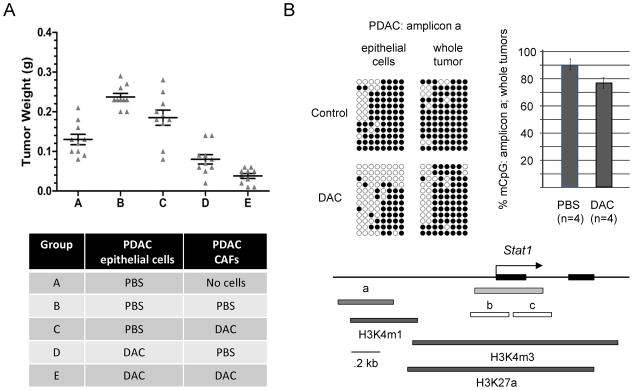
A, Short term cultures of pancreatic CAFs or PDAC epithelial cells from the KPC-Brca1 tumors were pre-treated with DAC or grown without the drug, followed by mixing of the two types of cells and allografting into the flanks of nude mice. Mice were sacrificed after 3 weeks and the tumor diameters were measured. As indicated by comparing condition (A) to condition (B), the presence of CAFs promotes tumor growth. As indicated by comparing condition (B) to condition (C), pre-treating the carcinoma cells alone significantly reduces the growth of the tumor allografts. As indicated by comparing condition (D) to condition (E), treating the CAFs as well before mixing them with the malignant epithelial cells leads to the greatest net anti-tumor effect. B, Bisulfite sequencing of genomic DNA from PDAC tumor cells exposed to 0 or 0.5 μM DAC in short-term culture, and whole PDAC tumors from mice receiving PBS vehicle control or DAC shows partial demethylation of a potential regulatory region overlapping a block of histone H3K4m1 chromatin modification (from ENCODE/LICR fetal liver ChIP-Seq data) extending from approximately 0.6 to 1kb upstream of the STAT1 first exon. The bar graph summarizes the bisulfite sequencing data (% methylated CpG’s in amplicon a) for the indicated number of in vivo-treated tumors analyzed. Bisulfite sequencing of the two amplicons (b, c) overlapping the STAT1 CpG island (green) showed nearly complete lack of CpG methylation in both DAC-treated and untreated tumor samples (data not shown).
