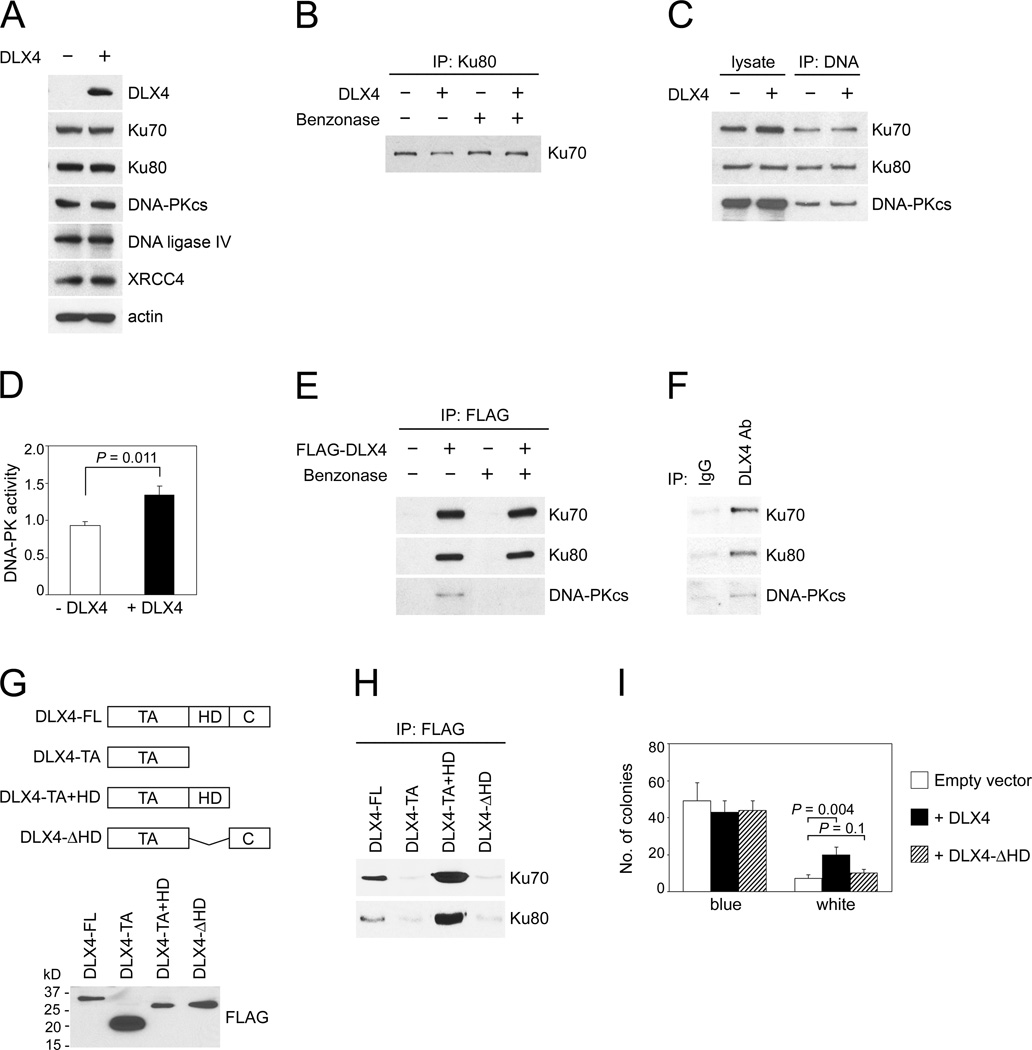
Fig. 4. DLX4 interacts with the NHEJ machinery and stimulates DNA-PK activity.
[A] Western blot of levels of canonical NHEJ components in vector-control (-DLX4) and +DLX4 U2OS cells. [B] Interaction of Ku80 with Ku70 was assayed by IP in extracts treated with benzonase to remove DNA or left untreated. [C] Interactions of Ku proteins and DNA-PKcs with DNA ends were assayed by incubating biotinylated oligomer duplexes with U2OS nuclear extracts. Following DNA pull-down, DNA-associated proteins were analyzed by immuno-blotting. [D] DNA-PK activity in U2OS nuclear extracts (expressed as pmol ATP/minute/µg of protein). [E] Interactions of FLAG-tagged DLX4 with Ku70, Ku80 and DNA-PKcs were assayed in U2OS whole cell extracts (treated with benzonase or left untreated) by IP using FLAG Ab and immunoblotting using Abs to NHEJ components. [F] Interactions of endogenous DLX4 with Ku proteins and DNA-PKcs were assayed by IP in TOV112D extracts (not treated with benzonase). [G] Western blot of FLAG-tagged proteins containing full-length DLX4 (FL), transactivation domain (TA), homeodomain (HD) and C-terminal tail (C) expressed in U2OS cells. [H] Interactions of FLAG-tagged DLX4 proteins with Ku proteins were detected by IP as in [E]. [I] Colony formation in end-joining assays using extracts of U2OS cells that were transfected with empty vector, full-length DLX4 and a truncated form lacking the Ku-interacting domain (DLX4-ΔHD). Shown are average results of 3 independent experiments.