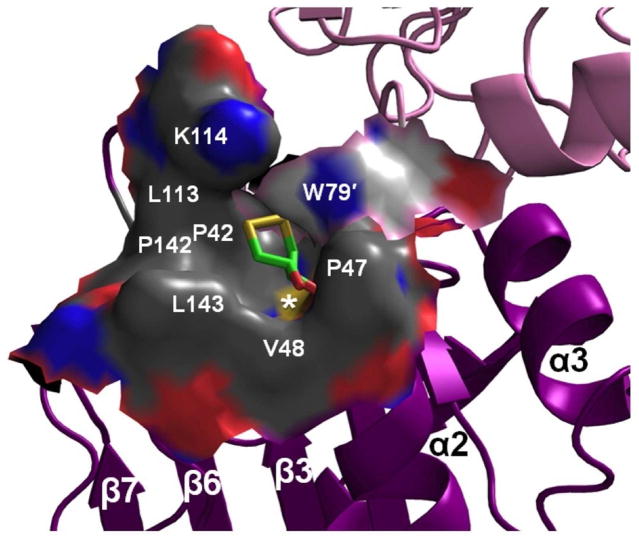
Figure 3.
The ApPrxQ FF active site hydrophobic collar. Shown is the active site molecular surface annotated with residue numbers and showing the bound DTT (sticks). The surface is colored by element, distinguishing carbons from the main monomer (dark gray) or the other subunit (light gray), nitrogens (blue), oxygens (red), and sulfurs (yellow). The peroxidatic Cys thiolate surface is marked by a white asterisk (*). The catalytic Arg122 contributes the blue nitrogen surface left of the asterisk, and barely visible red surface right of the asterisk is from the Ser46 hydroxyl.