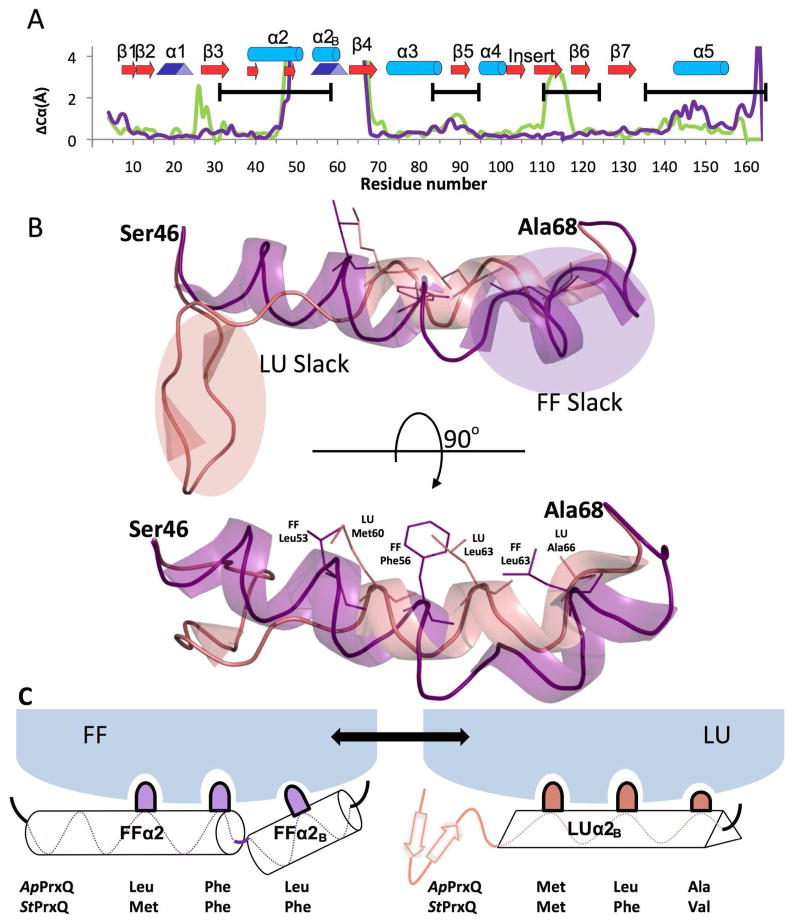
Figure 5.
Essential structural features of the PrxQ CRα2 FF↔LU transition. (A) Cα shifts between the FF and LU conformations are given for the ApPrxQ pair (purple) and the StPrxQ/SsPrxQ4 pair (green). ApPrxQ numbering is used and shifts <4 Å are shown in the inset. Black bars indicate the regions undergoing intermediate exchange in the AtPrxQ NMR study37. Peaks near residues 25 and 110 in the StPrxQ/SsPrxQ4 pair are not related to the FF/LU transition, but due, respectively, to a one residue insertion in StPrxQ4 and the influence of crystal contacts. (B) Orthogonal views of the backbone ribbon for residues 46 – 68 in the FF (purple) and LU (salmon) conformations. Side chains for the hydrophobic anchors are shown as sticks (labeled in the lower view) and regions in each conformation not well anchored and providing slack that extends as part of the conformational change are indicated in the upper view. (C) Schematic model for how the hydrophobic anchors shift interactions with the protein core during the conformation change.