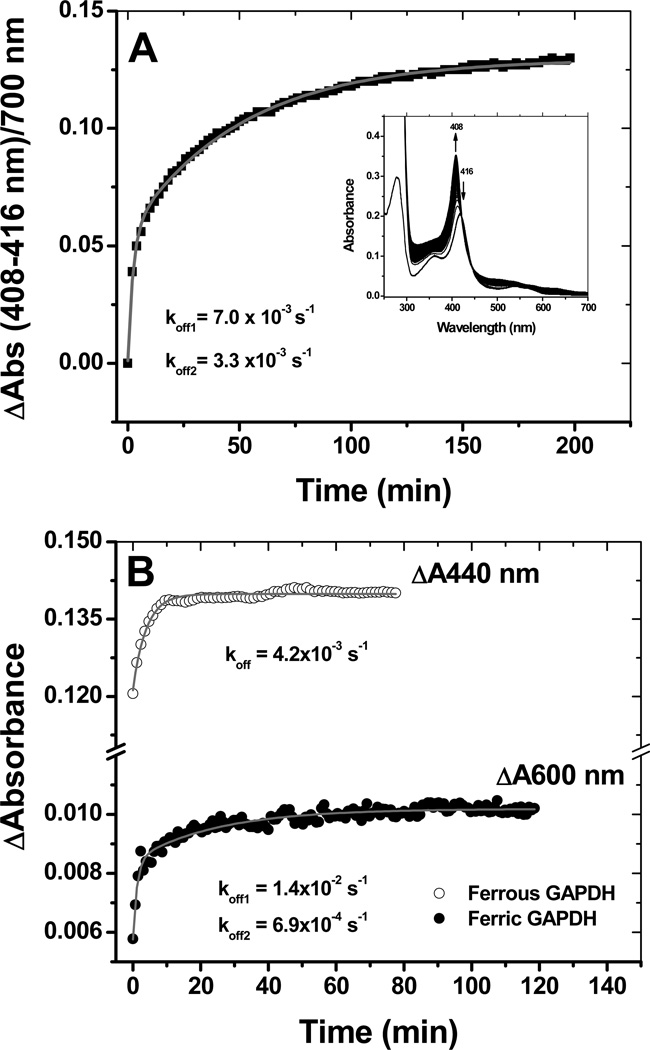
Figure 5.
Heme transfer from GAPDH to apomyoglobin. Heme binding to GAPDH is a reversible process. Incubation of ferric GAPDH-heme with a 5-fold excess of apo-myoglobin resulted in the stoichiometric transfer of heme with rates of 7.0 ×10−3 s−1 and 3.3 ×10−4 s−1 at 10 °C (bi-exponential fit) (Panel A). Time courses for ferric and ferrous heme transfer to apomyoglobin at 25 °C (Panel B). Heme transfer from ferrous GAPDH to apo-myoglobin was derived from the absorption changes at 440 nm (maximum absorption for ferrous Mb) and was best fit to a single exponential equation. In panel B, heme transfer from ferric GAPDH to apo-myoglobin was derived from the absorption changes at 600 nm (charge transfer band of holo-Mb) to give koff rates of 1.4 ×10−2 s−1 and 6.9 ×10−3 s−1.