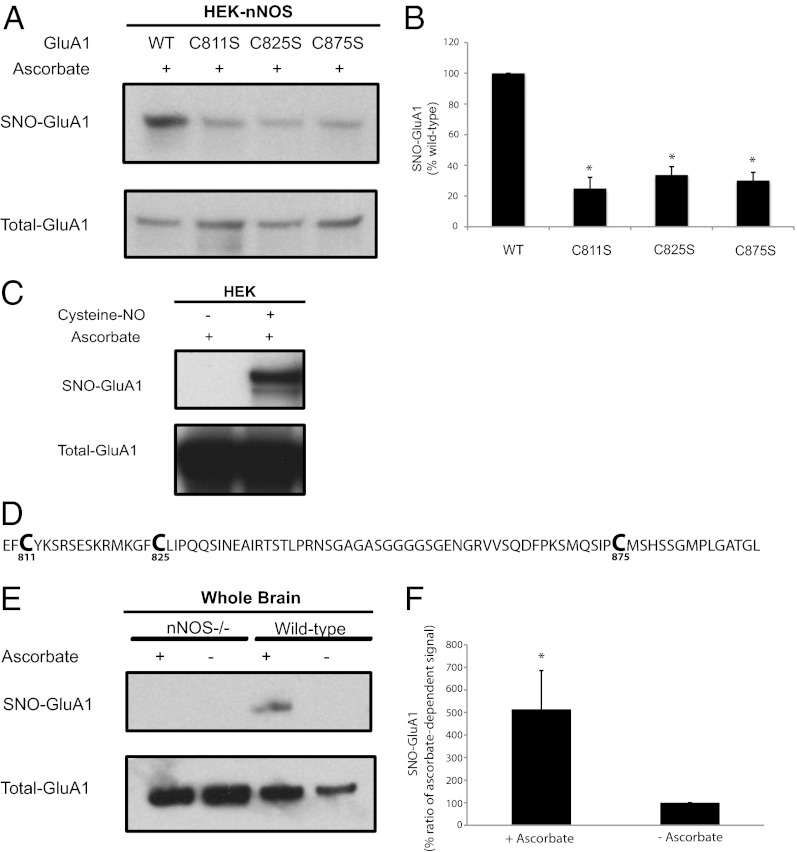
Fig. 1.
Endogenous S-nitrosylation of GluA1. (A) HEK-nNOS cells stably expressing nNOS were transfected with WT GluA1 or cysteine mutants of GluA1, and lysates were analyzed by the biotin-switch assay. (B) Quantification of experiments in A. (C) HEK cells transfected with GluA1 were treated with 100 µM of cysteine-NO for 10 min, and lysates later were analyzed by the biotin-switch assay, which demonstrates the presence of S-nitrosylated cysteines. (D) Amino acid sequence of the cytosolic C-terminal tail of GluA1. (E) Brain lysates obtained from WT or nNOS knockout (nNOS−/−) animals were analyzed by the biotin-switch assay to determine physiologic nitrosylation of GluA1. The ascorbate dependence demonstrates specifically the presence of S-nitrosylation. (F) Quantification of ascorbate-dependent signal for nitrosylated-GluA1 in WT animals. Data are means ± SEM, P < 0.05. All Western blots show representative images of experiments performed at least three times.