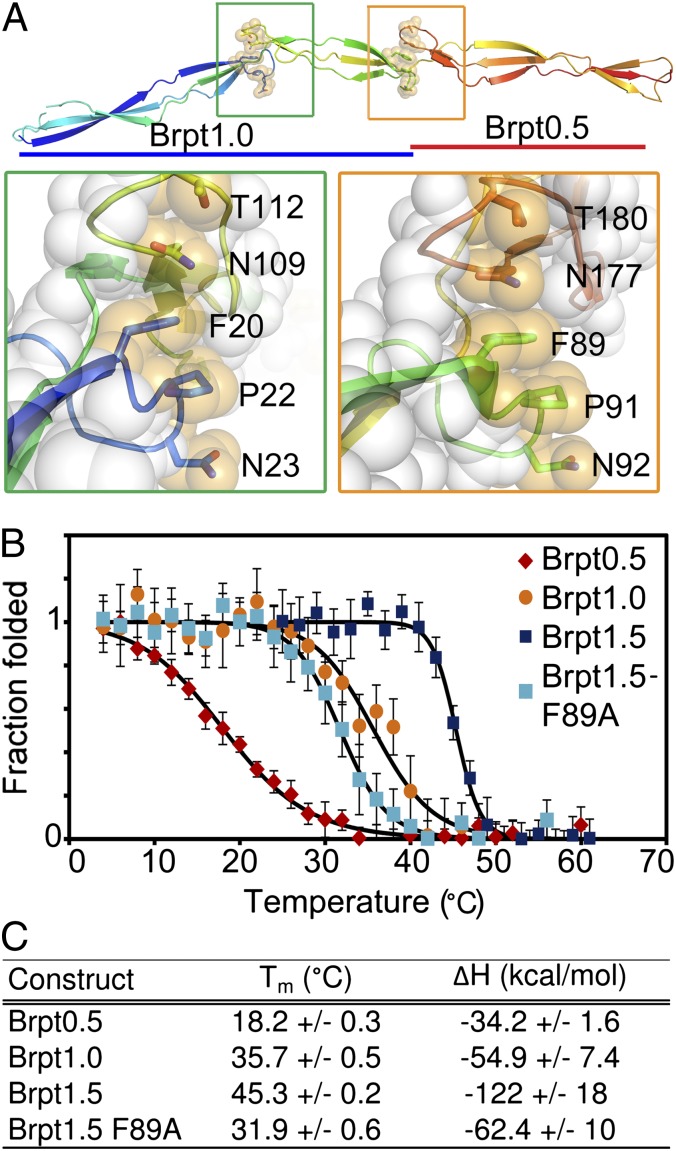
Fig. 3.
Stability of the freestanding β-sheet fold. (A) Brpt1.5 structure, with the side chains of the hydrophobic stacking residues shown as sticks (colored per backbone) overlaid with transparent spheres (orange). (Left Inset, green box) The hydrophobic stack between the N-terminal G5 domain (blue residues) and spacer domain (yellow residues). (Right Inset, orange box) The interaction between the spacer domain (green residues) and C-terminal G5 domain (orange residues). In both insets the atoms of the remaining residues are shown as transparent white spheres. (B) Circular dichroism thermal denaturation data from Brpt0.5 (red), Brpt1.0 (orange), Brpt1.5 (blue), and the Brpt1.5-F89A mutant (cyan). All melts were fully reversible. The fits to a model of two-state thermal denaturation are superimposed (black). (C) Tm and enthalpy change (ΔH) determined from the thermal denaturation data.