Fig. 2. Antioxidant function of GSH.
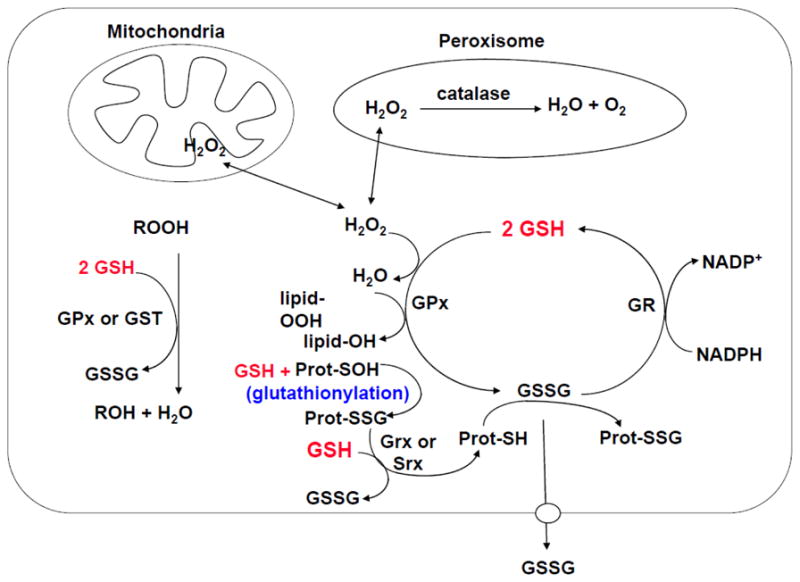
Aerobic metabolism generates hydrogen peroxide (H2O2), which can be metabolized by GSH peroxidase (GPx) in the cytosol and mitochondria, and by catalase in the peroxisome. GSSG can be reduced back to GSH by GSSG reductase (GR) at the expense of NADPH, thereby forming a redox cycle. Organic peroxides (ROOH) can be reduced by either GPx or GSH S-transferase (GST). GSH also plays a key role in protein redox signaling. During oxidative stress, protein cysteine residues can be oxidized to sulfenic acid (Prot-SOH), which can react with GSH to form protein mixed disulfides Prot-SSG (glutathionylation), which in turn can be reduced back to Prot-SH via glutaredoxin (Grx) or sulfiredoxin (Srx). This is a mechanism to protect sensitive protein thiols from irreversible oxidation and may also serve to prevent loss of GSH under oxidative conditions. The ability of the cell to reduce GSSG to GSH may be overcome during severe oxidative injury, leading to an accumulation of GSSG. To prevent a shift in the redox equilibrium, GSSG can either be actively transported out of the cell or react with a protein sulfhydryl (Prot-SH) to form a mixed disulfide (Prot-SSG).
